Text Solution
Verified by Experts
Topper's Solved these Questions
CARBON AND ITS COMPOUNDS
OSWAAL PUBLICATION|Exercise NCERT Corner (Intext Questions)|13 VideosCARBON AND ITS COMPOUNDS
OSWAAL PUBLICATION|Exercise NCERT Corner (Textbook Exercises)|15 VideosCARBON AND ITS COMPOUNDS
OSWAAL PUBLICATION|Exercise Topic II- Carbon Compounds , Soap and Detergents (Short Answer Type Question-II)|30 VideosACIDS,BASES AND SALTS
OSWAAL PUBLICATION|Exercise NCERT CORNER TEXTBOOK EXERCISES|15 VideosCHEMICAL REACTIONS AND EQUATIONS
OSWAAL PUBLICATION|Exercise NCERT CORNER (Textbook Exercises)|20 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-CARBON AND ITS COMPOUNDS-Topic II- Carbon Compounds , Soap and Detergents (Long Answer Type Questions)
- (a) What is isomerism ? Name the isomers of butane. (b) Name the air...
Text Solution
|
- (a) The boxes given here with numbers 1, 2, 3 and 4 represent a class ...
Text Solution
|
- (a) How will you bring about following reactions? Write the concerned ...
Text Solution
|
- Write balanced chemical equation for the following: (i) Methane is b...
Text Solution
|
- (a) Complete the following equations : (i) CH(3)COOH + KHCO(3)overse...
Text Solution
|
- (a) Complete the following equations : (i) nCH(2) = CH(2) rightarrow...
Text Solution
|
- (i) "Covalent compounds have low melting and boiling points". Justify ...
Text Solution
|
- Describe the additon reaction of carbon compounds with its application...
Text Solution
|
- (i) You have three unlabelled test-tubes containing ethanol, ethanoic ...
Text Solution
|
- A carbon compound on heating with excess cond. H(2)SO(4) forms another...
Text Solution
|
- Both soap and detergent are same type of salts. What is the difference...
Text Solution
|
- An organic compound A is widely used as a preservative in pickles and ...
Text Solution
|
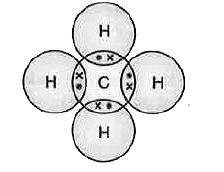
- Make the structure of methane by showing sharing of electrons between ...
Text Solution
|
- (i) Write the name of the following compounds. CH(3)CH(2)COOH,C(6)H(...
Text Solution
|
- An organic compound is widely used as a preservative in pickles and ha...
Text Solution
|