Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 1 (Long answer questions)|2 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 1 (Short answer questions )|8 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise CONVERSIONS|51 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise Exercise 3.2|41 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALCOHOLS, PHENOLS AND ETHERS-PROBLEM
- How many structural isomers exist with the formula C(4)H(10)O?
Text Solution
|
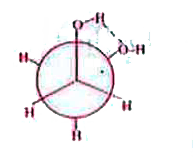
- Identify the most stable conformer of glycol.
Text Solution
|
- How many amyl alcohol structures are possible ?
Text Solution
|
- Arrange the following set of compounds in order of their increasing bo...
Text Solution
|
- Give IUPAC names of the following CH3 - undersetoverset(|)(Cl)(CH...
Text Solution
|
- Give IUPAC names of the CH(3)-underset(CH(3))underset(|)CH-O-CH(2)CH(3...
Text Solution
|
- Give IUPAC names of the following
Text Solution
|
- How acetylene is converted to ethyl alcohol ?
Text Solution
|
- How benzyl alcohol is obtained from benzyl chloride ?
Text Solution
|
- How is n-propyl alcohol prepared from propene ?
Text Solution
|
- Suggest a method for the conversion of CH(3)-CH(2)-NH(3) into ethyl al...
Text Solution
|
- CH(3)-underset(CH(3))underset(|)CH-CH=CH(2)+H(2)O overset(H^(+))toX ...
Text Solution
|
- How is methyl alcohol converted to ethyl alcohol?
Text Solution
|
- Give the structures and IUPAC names of the products expected from the ...
Text Solution
|
- Propane is treated with methyl magnesium bromide and them subjected to...
Text Solution
|
- Show how are the following alcohols prepared by the reaction of a suit...
Text Solution
|
- Write structures of the products of the following reactions : CH3 ...
Text Solution
|
- Write structures of the products of the following reactions :
Text Solution
|
- Write structures of the products of the following reactions : CH3 ...
Text Solution
|
- tert-Butyl alcohol is much more soluble in water than n-butyl alcohol....
Text Solution
|