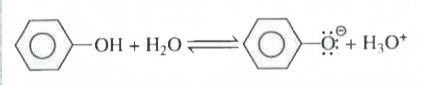
Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 1 (Long answer questions)|2 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE - 1 (Short answer questions )|8 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise CONVERSIONS|51 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise Exercise 3.2|41 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALCOHOLS, PHENOLS AND ETHERS-PROBLEM
- What happens when ethyl alcohol is treated with HI and red phosphorous...
Text Solution
|
- What happens when one drinks ethanol mixed with methanol? Why?
Text Solution
|
- Phenol is treated with C Cl(4) in presence of NaOH. What happens ?
Text Solution
|
- What is the reason for the formation of tribromo phenol when phenol is...
Text Solution
|
- Write the electrophile and intermediate in Reimer-Tiemann reaction.
Text Solution
|
- Write the decreasing order of acidic strengths of the following: A :...
Text Solution
|
- Write the structures of the major products expected from the following...
Text Solution
|
- Predict the major products formed from the following reactions : a) ...
Text Solution
|
- Arrange the following compounds in decreasing order of their acidic st...
Text Solution
|
- What is the weight of bromine required to give 0.1 mole of a white pre...
Text Solution
|
- Chloroethane, 2-chloropropane and chloroethene are treated with methox...
Text Solution
|
- Give one example of an ether which cannot be decomposed by HI.
Text Solution
|
- Give the major products that are formed by heating each of the followi...
Text Solution
|
- The following is not an appropriae reaction for the preparation of t-b...
Text Solution
|
- Ether can be dried over sodium, but not alcohol. Why?
Text Solution
|
- How is ethyl iso-propyl ether prepared ?
Text Solution
|
- What product is expected when tertiary butyl bromide is reacted with s...
Text Solution
|
- How is ether distinguished from ethanol using iodine and alkali ?
Text Solution
|
- Why anisole does not give iodobenzene with excess HI ?
Text Solution
|
- The following is not an appropriate reaction for the preparation of t-...
Text Solution
|