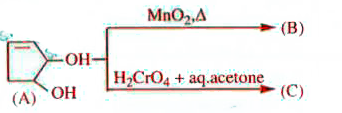A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise ETHERS (OBJECTIVE EXERCISE-1 (INTRODUCTION AND PREPARATIONS))|14 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise ETHERS (OBJECTIVE EXERCISE-1 (PROPERTIES))|14 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise PHENOLS (OBJECTIVE EXERCISE - 2A)|30 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise Exercise 3.2|41 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALCOHOLS, PHENOLS AND ETHERS-PHENOLS (OBJECTIVE EXERCISE - 2B)
Text Solution
|
Text Solution
|
- The most unlikely representation of resonance structure of P-nitrophen...
Text Solution
|
- Which one of the following is most acidic?
Text Solution
|
- Among the following the pKa is minimum for
Text Solution
|
- In which of the following molecules - NO2 group shows only I effect
Text Solution
|
- The major product X is
Text Solution
|
- The correct representation of single carbene is
Text Solution
|
- Dinitration of 3-methyl-phenol gives the following major product.
Text Solution
|
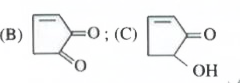
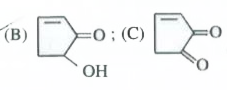
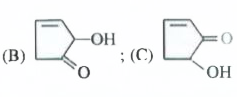
- The compounds B and C , respectively are
Text Solution
|