Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES
DC PANDEY ENGLISH|Exercise Level 2 Single Correct|12 VideosTHERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES
DC PANDEY ENGLISH|Exercise Level 2 More Than One Correct|7 VideosTHERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES
DC PANDEY ENGLISH|Exercise Level 1 Objective|10 VideosTHERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY ENGLISH|Exercise Medical entrance gallary|30 VideosUNIT AND DIMENSIONS
DC PANDEY ENGLISH|Exercise Assertion And Reason|2 Videos
DC PANDEY ENGLISH-THERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES-Level 1 Subjective
- A compressor pumps 70 L of air into a 6 L tank with the tempertaure re...
Text Solution
|
- A partially inflated balloon contains 500 m^3 of helium at 27^@ C and ...
Text Solution
|
- A cylinder whose inside diameter is 4.00 cm contains air compressed by...
Text Solution
|
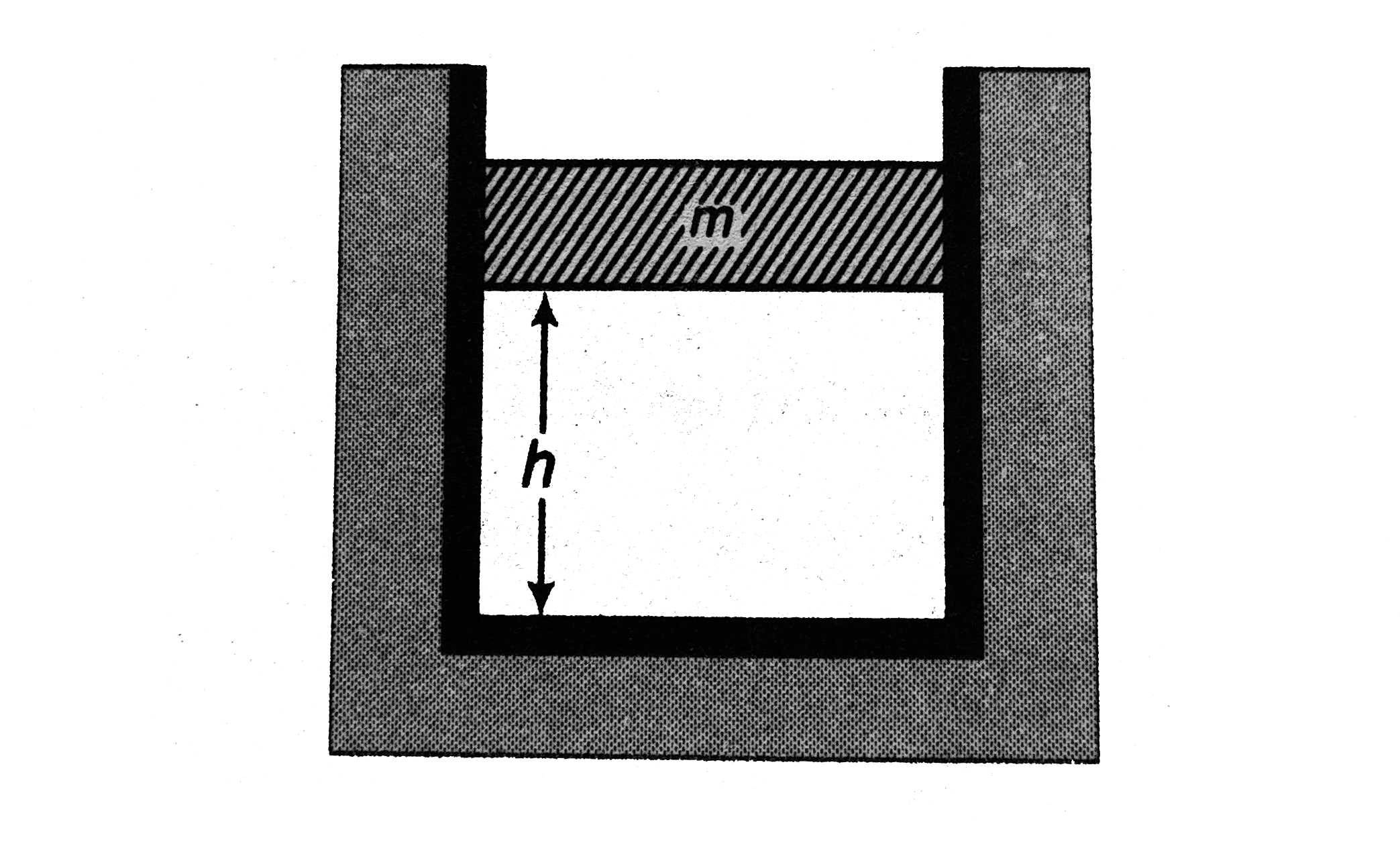
- The closed cylinder shown in figure has a freely moving piston separat...
Text Solution
|
- Two gases occupy two containers (A) and (B). The gas in (A) of volume ...
Text Solution
|
- A glass bulb of volume 400 cm^(3) is connected to another bulb of volu...
Text Solution
|
- The condition called standard temperature and pressure (STP) for a gas...
Text Solution
|
- A large cylindrical tank contains 0.750 m^3 of nitrogen gas at 276@ C ...
Text Solution
|
- A vessel of volume 5 litres contains 1.4 g of N2 and 0.4 g of He at 15...
Text Solution
|
- Temperature of diatomic gas is 300 K. If moment of intertia of its mol...
Text Solution
|
- Find the number of degrees of freedom of molecules in a gas. Whose mol...
Text Solution
|
- In a certain (2/5) th of the energy of molecules is associated with th...
Text Solution
|
- A mixture contains 1 mole of helium (Cp = 2.5 R, Cv= 1.5 R. ) and 1 m...
Text Solution
|
- An ideal gas (Cp / Cv = gamma) is taken through a process in which the...
Text Solution
|
- An ideal gas is taken through a process in which the pressure and the ...
Text Solution
|
- The pressure of a gas in a 100 mL container is 200 kPa and the. Averag...
Text Solution
|
- One gram mole NO2 "at" 47^@ C and 2 atm pressure in kept in a vessel. ...
Text Solution
|
- A 2.00 mL volume container contains 50 mg of gas at a pressure of 100 ...
Text Solution
|
- Call the (rms) speed of the molecules in an ideal gas V(0) at temperat...
Text Solution
|
- (a) What is the average translational kinetic energy of a molecule of ...
Text Solution
|
 .
.