Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-LAWS OF THERMODYNAMICS-Level 2 Subjective
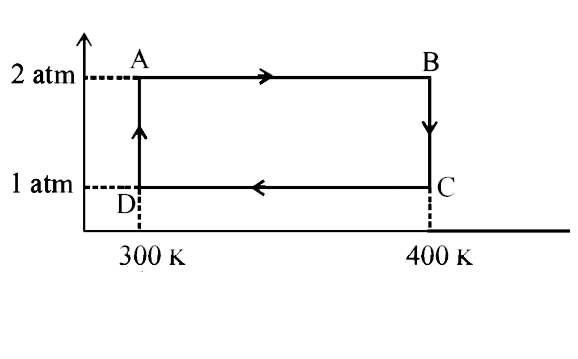
- Two moles of helium gas undergo a cyclic process as shown in Fig. Assu...
Text Solution
|
- 1.0 k-mol of a sample of helium gas is put through the cycle of operat...
Text Solution
|
- The density (rho) versus pressure (p) graph of one mole of an ideal mo...
Text Solution
|
- An ideal gas goes through the cycle abc. For the complete cycle 800J o...
Text Solution
|
- A cylinder of ideal gas is closed by an 8kg movable piston of area 60c...
Text Solution
|
- Three moles of an ideal gas (Cp=7/2R) at pressure p0 and temperature T...
Text Solution
|
- Two moles of helium gas(lambda=5//3) are initially at temperature 27^@...
Text Solution
|
- An ideal monoatomic gas is confined in a cylinder by a spring-loaded p...
Text Solution
|
- An ideal diatomic gas (gamma=7/5) undergoes a process in which its int...
Text Solution
|
- For an ideal gas the molar heat capacity varies as C=CV+3aT^2. Find th...
Text Solution
|
- One mole of an ideal monatomic gas undergoes the process p=alphaT^(1//...
Text Solution
|
- One mole of a gas is put under a weightless piston of a vertical cylin...
Text Solution
|
- An ideal monatomic gas undergoes a process where its pressure is inver...
Text Solution
|
- The volume of one mode of an ideal gas with adiabatic exponent gamma i...
Text Solution
|
- Two moles of a monatomic ideal gas undergo a cyclic process ABCDA as s...
Text Solution
|
- Pressure p, volume V and temperature T for a certain gas are related b...
Text Solution
|
- An ideal gas has a specific heat at constant pressure Cp=(5R)/(2). The...
Text Solution
|
- Three moles of an ideal gas being initially at a temperature Ti=273K w...
Text Solution
|