Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MODERN PHYSICS - 1
DC PANDEY ENGLISH|Exercise Exercise 33.4|8 VideosMODERN PHYSICS - 1
DC PANDEY ENGLISH|Exercise Level -1 Assertion And Reason|10 VideosMODERN PHYSICS - 1
DC PANDEY ENGLISH|Exercise Exercise 33.2|12 VideosMODERN PHYSICS
DC PANDEY ENGLISH|Exercise Integer Type Questions|17 VideosMODERN PHYSICS - 2
DC PANDEY ENGLISH|Exercise Level 2 Subjective|10 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-MODERN PHYSICS - 1-Exercise 33.3
- if lambda(Cu) is the wavelength of Kalpha, X-ray line fo copper (atomi...
Text Solution
|
- Which one of the following statement is wrong in th econtext of X-rays...
Text Solution
|
- Kalpha wavelength emitted by an atom of atomic number Z=11 is lambda. ...
Text Solution
|
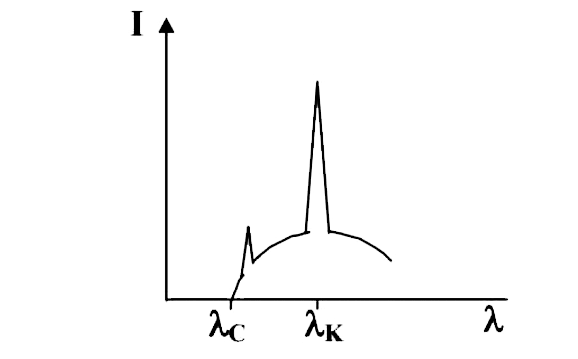
- The intensity of X- ray from a coolidge tube is plotted againest wavel...
Text Solution
|
- X-ray are produced in an X-ray tube operating at a given accelerating...
Text Solution
|
- Characteristic X-rays of frequency 4.2xx10^18 Hz are produced when tr...
Text Solution
|