A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-PROPERTIES OF MATTER-Integer
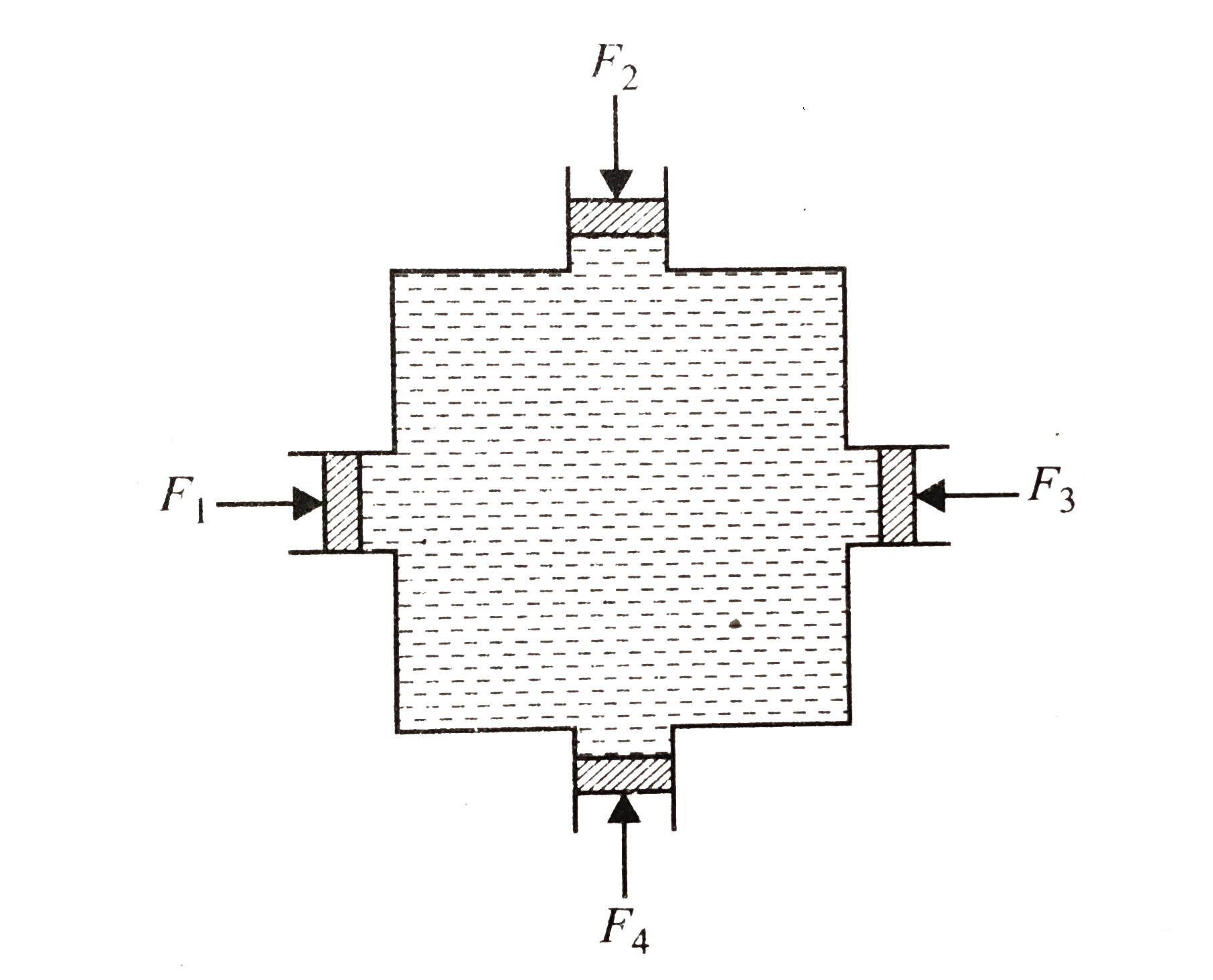
- Figure shows water filled in a symmetrical container. Four pistons of ...
Text Solution
|
- The reading of spring balance when a block is suspended from it in air...
Text Solution
|
- The two wires shown in figure are made of the same material which has ...
Text Solution
|
- A wooden cube of side 10 cm and density 0.8" gm/cm"^(3) is floating in...
Text Solution
|
- A ball of mass 1 kg falls from a height of 5 m above the free surface ...
Text Solution
|
- A log of wood of mass 120 Kg floats in water. The weight that can be p...
Text Solution
|
- A large block of ice 10 cm thick with a vertical hole drilled through ...
Text Solution
|
- A cubical block 'A' of mass m(0)(=a^(3)rho=3 kg) of edge 'a' and densi...
Text Solution
|
- A solid uniform ball having volume V and density rho floats at the int...
Text Solution
|