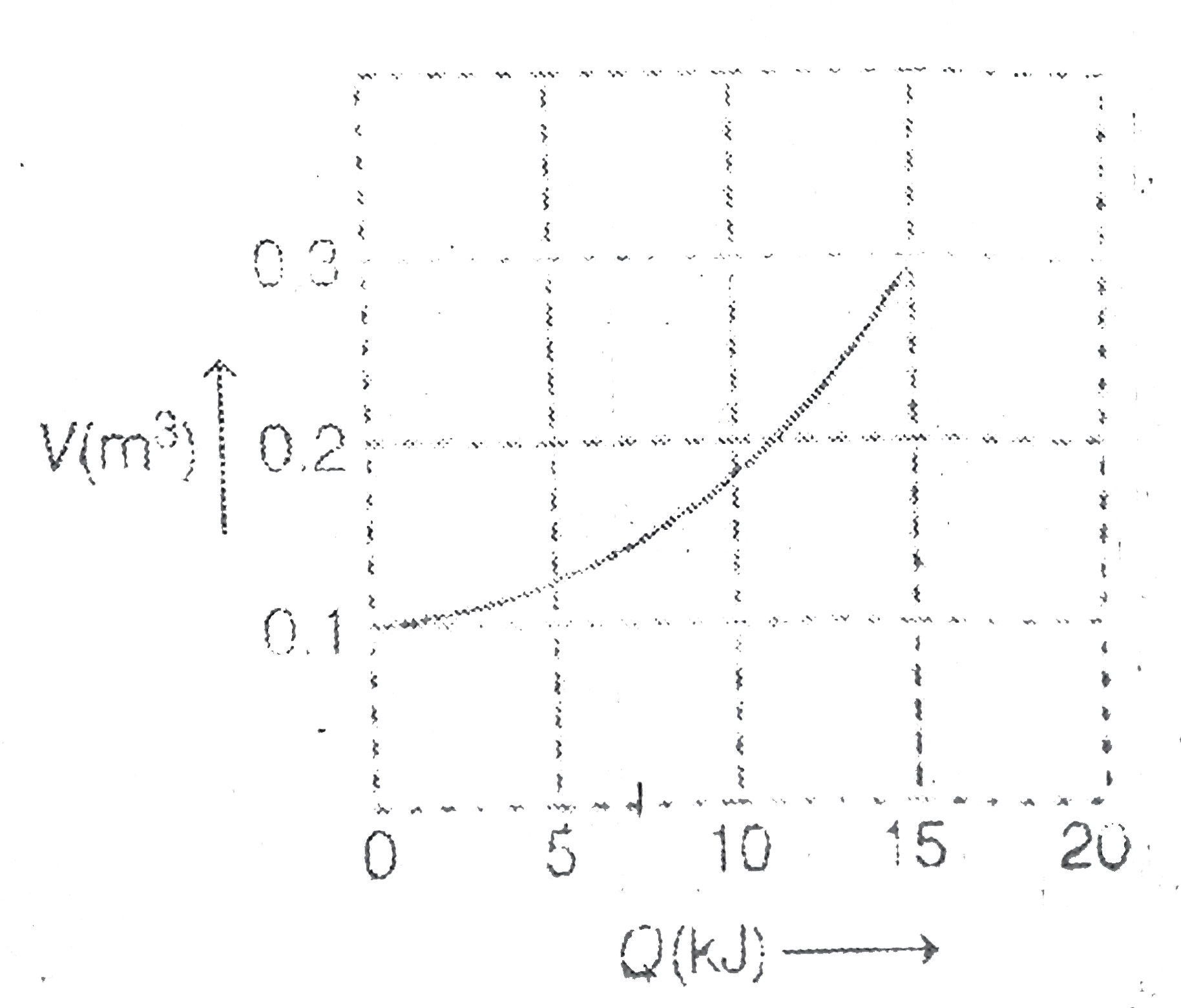
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-CURRENT ELECTRICITY-All Questions
- One mole of a diatomic ideal gas undergoes a cyclic process ABC as sho...
Text Solution
|
- A copper block of mass 2.5 kg is heated in a furnace to a temperature ...
Text Solution
|
- Consider isothermal expansion of 0.5 mole of an ideal gas when Q amoun...
Text Solution
|
- A brass boiler has a base area of 0.15m^2 and thickness 1.0 cm it boil...
Text Solution
|
- A monoatomic ideal gas is used in a carnot engine as the working subst...
Text Solution
|
- Two adiabatic containers have volumes V(1) and V(2) respectively. The ...
Text Solution
|
- A uniform metallic disc of radius r and mass m is spinning with angula...
Text Solution
|
- Three identical rods AB, CD and PQ are joined as shown. P and Q are mi...
Text Solution
|
- When the temprature of a gas filled in a closed vessel is increased by...
Text Solution
|
- If an ideal gas is heated at constant pressure :
Text Solution
|
- Two identical vessels A and B contain masses m and 2m of same gas. The...
Text Solution
|
- The following graphs shows two isothermal process for a fixed mass of ...
Text Solution
|
- A sample of ideal gas (gamma=1.4) is heated at constant pressure. If a...
Text Solution
|
- If 2 mol of an ideal monatomic gas at temperature T(0) are mixed with ...
Text Solution
|
- Heat is supplied to a diatomic gas at constant pressure. The ratio o...
Text Solution
|
- The energy density u/V of an ideal gas is related to its pressure P as
Text Solution
|
- A ring consisting of two parts ADB and ACB of same conductivity k carr...
Text Solution
|
- Three conducting rods of same material and cross-section are shown in ...
Text Solution
|
- Three rods of identical cross-sectional area and made from the same me...
Text Solution
|
- An ideal monoatomic gas undergoes a cyclic process ABCA as shown in th...
Text Solution
|