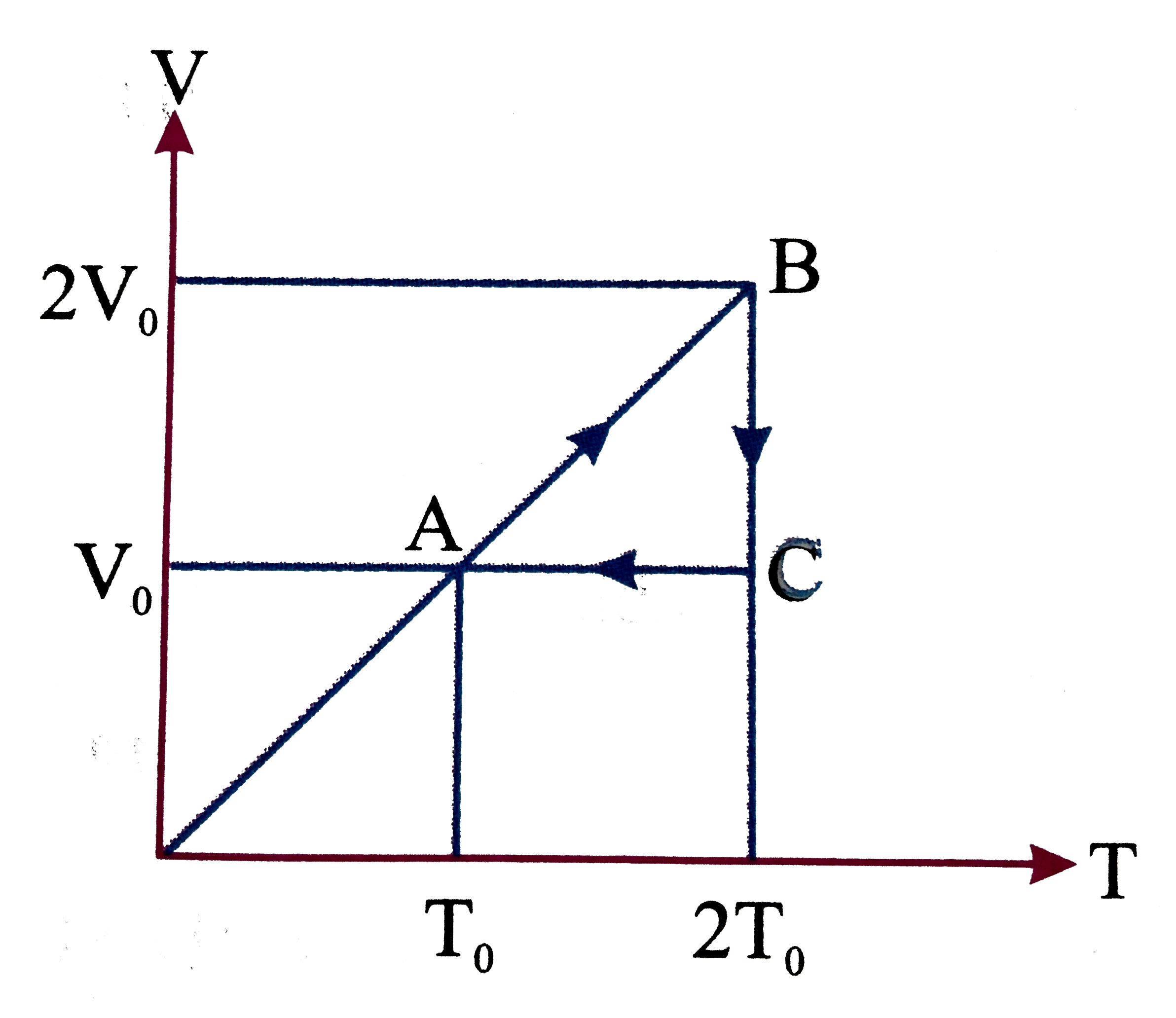
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-CURRENT ELECTRICITY-All Questions
- Three conducting rods of same material and cross-section are shown in ...
Text Solution
|
- Three rods of identical cross-sectional area and made from the same me...
Text Solution
|
- An ideal monoatomic gas undergoes a cyclic process ABCA as shown in th...
Text Solution
|
- Three moles of an ideal monoatomic gas performs a cyclic process as sh...
Text Solution
|
- A ideal gas (gamma=1.5) is expanded adiabatically. How many times has ...
Text Solution
|
- Three samples of the same gas A,B and C (gamma=3//2) have initially eq...
Text Solution
|
- Temperature of an ideal gas is 300 K. The change in temperature of the...
Text Solution
|
- 70 calories of heat is required to raise the temperature of 2 moles of...
Text Solution
|
- Consider the two insulating sheets with thermal resistance R(1) and R(...
Text Solution
|
- Four spheres A, B, C and D of different metals but all same radius are...
Text Solution
|
- Themperature of a body theta is slightly more than the temperature of ...
Text Solution
|
- A gas undergoes a change of state during which 100J of heat is supplie...
Text Solution
|
- Unit mass of liquid of volume V(1) completely turns into a gas of volu...
Text Solution
|
- Pressure versus density graph of an ideal gas is shown in figure
Text Solution
|
- How much heat energy should be added to a mixture of 10 g of hydrogen...
Text Solution
|
- An ideal gas mixture filled inside a balloon expands according to the ...
Text Solution
|
- Ideal gas is taken through the process shown in the figure :
Text Solution
|
- Find V(A)-V(B) in steady state
Text Solution
|
- The specific heat of many solids at low temperatures varies with absol...
Text Solution
|
- Show that the volume thermal expansion coefficient for an ideal gas at...
Text Solution
|