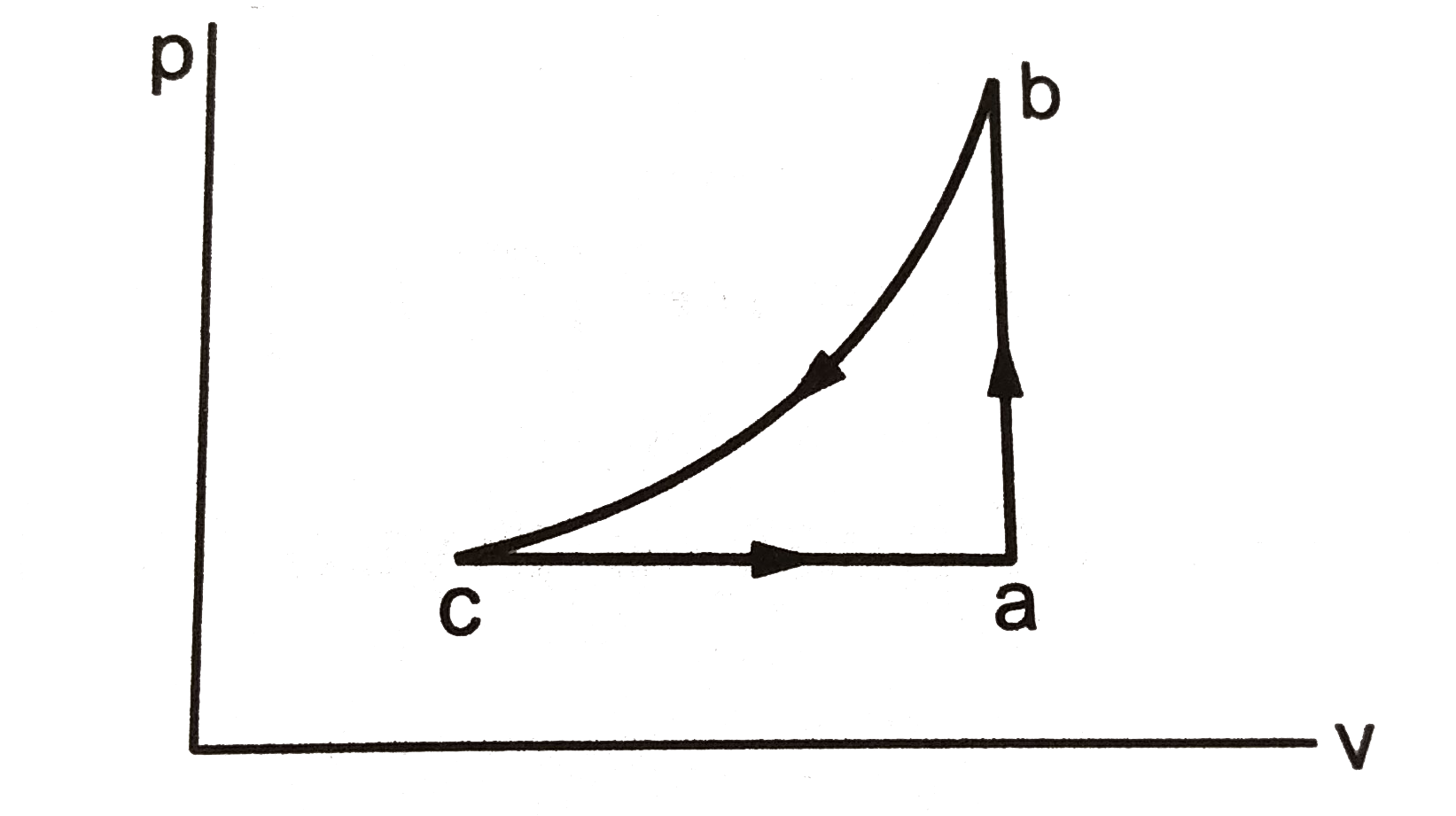
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-CURRENT ELECTRICITY-All Questions
- The molar heat capacity in a process of a diatomic gas if it does a wo...
Text Solution
|
- An insulator container contains 4 moles of an ideal diatomic gas at te...
Text Solution
|
- A sample of an ideal gas is taken through the cyclic process abca . It...
Text Solution
|
- Ideal monoatomic gas is taken through a process dQ = 2dU. Find the mol...
Text Solution
|
- n moles of an ideal monatomic gas undergo a proces in which the temper...
Text Solution
|
- One mole of a monoatomic ideal gas undergoes the process ArarrB in the...
Text Solution
|
- A monoatomic gas undergoes a process given by 2dU+3dW=0, then what is ...
Text Solution
|
- Two sheets of thickness d and 3d, are touching each other. The tempera...
Text Solution
|
- When an ideal diatomic gas is heated at constant pressure, the fractio...
Text Solution
|
- Two metallic spheres S1 and S2 are made of the same material and have ...
Text Solution
|
- The intensity of radiation emitted by the sun has its maximum value at...
Text Solution
|
- The average translational kinetic energy of O(2) (molar mass 32) molec...
Text Solution
|
- A vessel contains 1 mole of O2 gas (relative molar mass 32) at a tempe...
Text Solution
|
- rho-T equation of a gas in adiabatic process is given by
Text Solution
|
- 10 g of ice at 0^(@)C is mixed with m mass of water at 50^(@)C. What i...
Text Solution
|
- The ends of a copper rod of length 1m and area of cross-section 1cm^2 ...
Text Solution
|
- The heat capacity at constant volume of a monoatomic gas is 35 j//K. F...
Text Solution
|
- One mole of a monoatomic gas at 300K is mixed with two moles of diatom...
Text Solution
|
- A vessel of volume 3V contains a gas at pressure 4 P(0) and another ve...
Text Solution
|
- In a process V prop T^(2), temperature of 2 moles of a gas is increase...
Text Solution
|