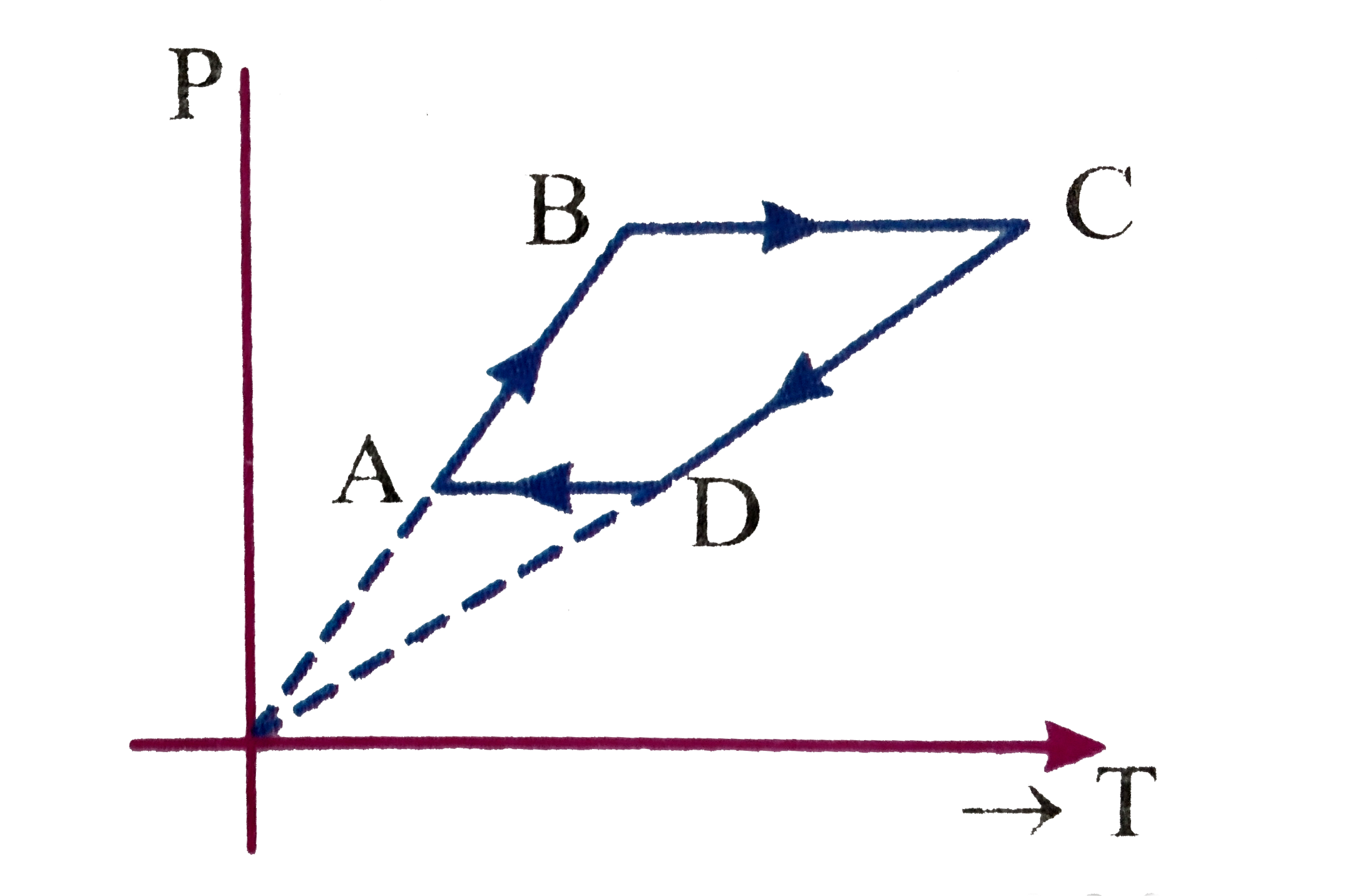
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-CURRENT ELECTRICITY-All Questions
- Figure illustrates a cycle conducted with n moles of an ideal gas. In ...
Text Solution
|
- One mole of a diatomic gas undergoes a process P = P(0)//[1 + (V//V(0)...
Text Solution
|
- 3 moles of an ideal mono atomic gas performs a cycle as shown in fig. ...
Text Solution
|
- Given T-p curve for three processes. Work done in process 1, 2 and 3 (...
Text Solution
|
- Figure shows two flasks connected to each other. The volume of the fla...
Text Solution
|
- A cylinder piston of mass M sides smoothlly inside a long cylinder clo...
Text Solution
|
- A 1-L flask contains some mercury. It is found that at different tempe...
Text Solution
|
- A point source of heat of power P is placed at the centre of a spheric...
Text Solution
|
- A fixed chamber isolated from surrounding is divided into equal halves...
Text Solution
|
- Figure shows the VT diagram for helium gas in a cyclic process. Find t...
Text Solution
|
- The state of an ideal gas is changed through an isothermal process at ...
Text Solution
|
- An ideal gas undergoes a process in which co-efficient of volume expan...
Text Solution
|
- A solid body X of very large heat capacity is kept in an atmosphere wh...
Text Solution
|
- A black body emits radiation at the rate P when its temperature is T. ...
Text Solution
|
- Where does a body weigh more – at the surface of the earth or in a min...
Text Solution
|
- A vertical cylinder of cross-section area A contains one mole of an id...
Text Solution
|
- Two spheres A and B have radius but the heat capacity of A is greater ...
Text Solution
|
- A thermally insulated chamber of volume 2V(0) is divided by a friction...
Text Solution
|
- Choose the correct option: In the arrangement shown in Fig. gas is t...
Text Solution
|
- P - V diagram of a cyclic process ABCA is as shown in Fig. Choose the ...
Text Solution
|