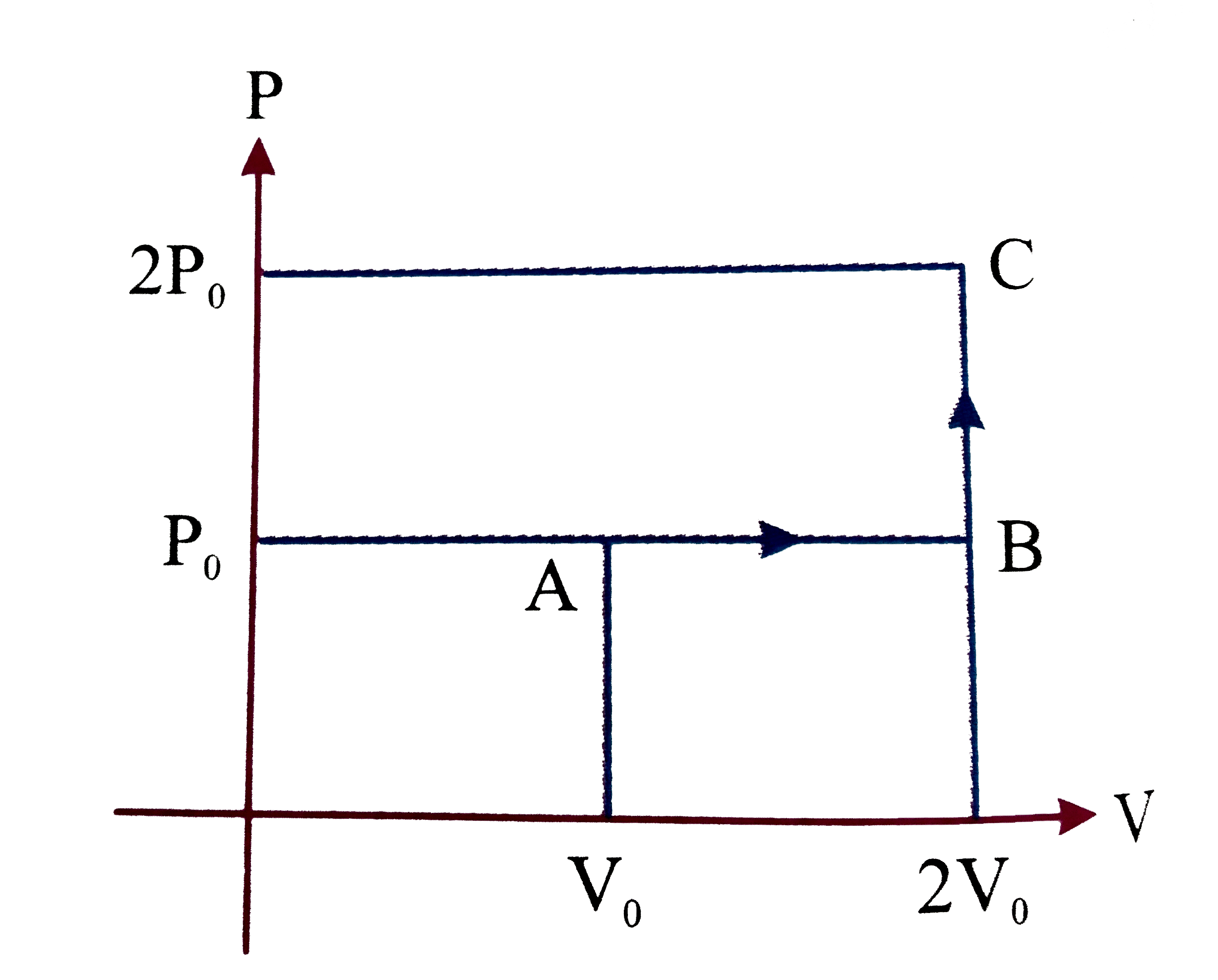
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-CURRENT ELECTRICITY-All Questions
- Internal energy of an ideal diatomic gas at 300 K is 100 J. In this 10...
Text Solution
|
- An ideal gas is allowed to expand in vacuum in rigid insulator contain...
Text Solution
|
- One mole of an ideal monoatomaic gas is taken from A to C along the pa...
Text Solution
|
- The temperature drop through a two layer furnace wall is 900^@C. Each ...
Text Solution
|
- n moles of a monoatomic gas undergoes a cyclic process ABCDA as shown....
Text Solution
|
- At ordinary temperatures, the molecules of an ideal gas have only tran...
Text Solution
|
- 1 kg of ice at 0^(@)C is mixed with 1.5 kg of water at 45^(@)C [latent...
Text Solution
|
- A vessel contains 1 mole of O(2) gas (molar mass 32) at a temperature ...
Text Solution
|
- In a thermodynamic process helium gas obeys the law TP^(-2//5) = const...
Text Solution
|
- The ednsity (rho) of an ideal gas varies with temperature T as shown i...
Text Solution
|
- A gas is found to obey the law P^(2)V= constant. The initial temperatu...
Text Solution
|
- n moles of an ideal gas undergo a process in which the temperature cha...
Text Solution
|
- One mole of an ideal monatomic gas (intial temperature T(0)) is made t...
Text Solution
|
- Two identical vessels contain helium and hydrogen gases at same temper...
Text Solution
|
- For an ideal gas :
Text Solution
|
- An ideal gas is taken from the state A (pressure p, volume V) to the s...
Text Solution
|
- From the following statements concerning ideal gas at any given temper...
Text Solution
|
- Which of the following quantities is the same for all ideal gases at t...
Text Solution
|
- During an experiment, an ideal gas is found to obey a condition (p^2)/...
Text Solution
|
- An ideal gas can be expanded form an initial state to a certain volume...
Text Solution
|