A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-CURRENT ELECTRICITY-All Questions
- During an experiment, an ideal gas is found to obey a condition (p^2)/...
Text Solution
|
- An ideal gas can be expanded form an initial state to a certain volume...
Text Solution
|
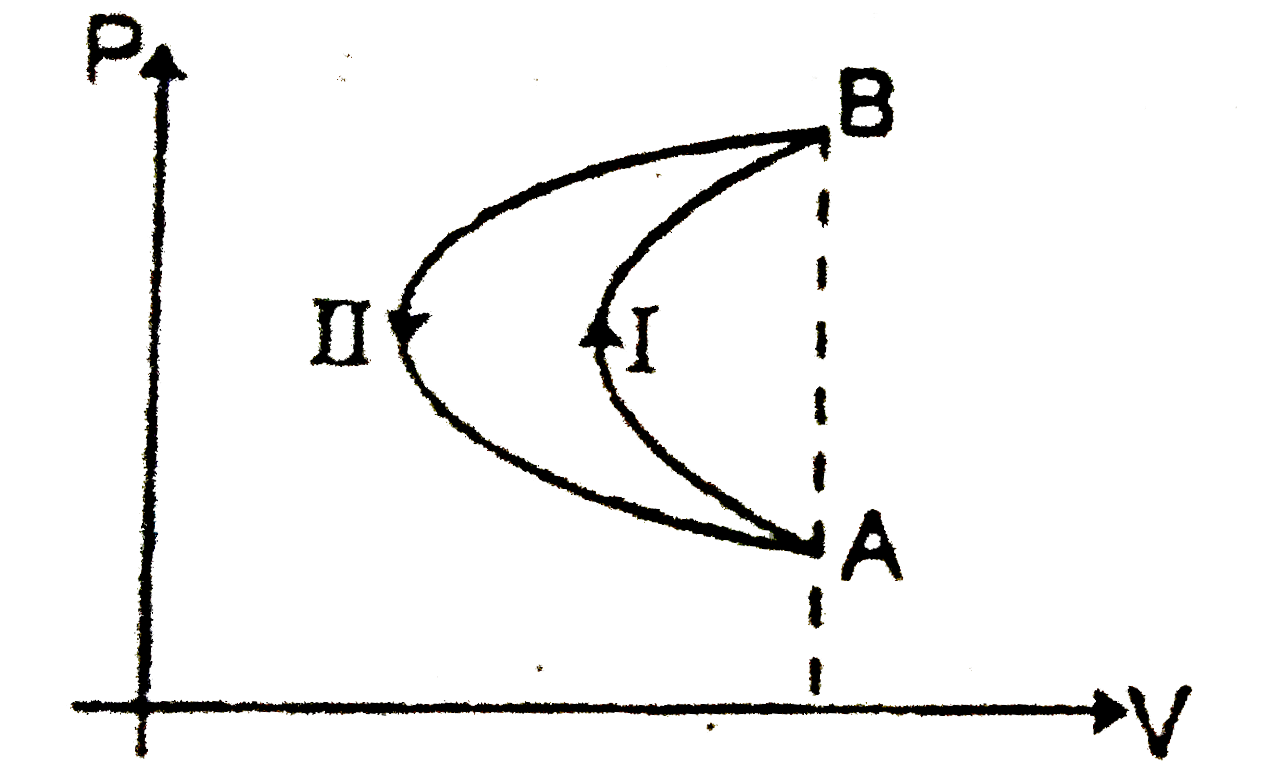
- In a cyclic process, a gas is taken from state A and B via path -I as ...
Text Solution
|
- During the melting of a slab of ice at 273K at atmospheric pressure,
Text Solution
|
- Tow large holes are cut in a metal sheet. If this is heated, distances...
Text Solution
|
- Two identical beakers with negligible thermal expansion are filled wit...
Text Solution
|
- A process is shown in the diagram. Which of the following curves may r...
Text Solution
|
- A student records Delta Q, Delta U and Delta W for a thermodynamic cyc...
Text Solution
|
- Two rods of different materials are placed between massive walls as sh...
Text Solution
|
- Two metal rods X and Y having equal cross-sectional areas are joined i...
Text Solution
|
- The graph below shows V-p curve for three processes. Choose the correc...
Text Solution
|
- One mole of an ideal gas undergoes a process such that P prop (1)/(T)....
Text Solution
|
- One mole of an ideal monoatomic gas is taken through a cyclic process ...
Text Solution
|
- Figure (Five straight line numbered 1, 2, 3, 4 and 5) shows graph of c...
Text Solution
|
- Two identical objects A and B (emissivity e(A) and e(B),e(A)ne e(B)) a...
Text Solution
|
- n mole of an ideal gas undergo an isothernal process at temperature T....
Text Solution
|
- Five rods of same material and same cross-section are joined as shown....
Text Solution
|
- Five rods of same material and same cross-section are joined as shown....
Text Solution
|
- graph of an ideal gas is as shown in figure. Work done by the ga...
Text Solution
|
- graph of an ideal gas is as shown in figure. Heat is supplied to ...
Text Solution
|