A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLVD PAPERS 2017 NEET, AIIMS & JIPMER
DC PANDEY ENGLISH|Exercise Solved Papers 2017(JIPMER)|28 VideosSOLVD PAPERS 2017 NEET, AIIMS & JIPMER
DC PANDEY ENGLISH|Exercise Solved paper 2018(NEET)|22 VideosSOLVD PAPERS 2017 NEET, AIIMS & JIPMER
DC PANDEY ENGLISH|Exercise Solved paper 2018(JIPMER)|38 VideosSIMPLE HARMONIC MOTION
DC PANDEY ENGLISH|Exercise Integer type questions|14 VideosSOUND WAVES
DC PANDEY ENGLISH|Exercise Exercise 19.7|4 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-SOLVD PAPERS 2017 NEET, AIIMS & JIPMER-Solved Papers 2017(AIIMS)
- What is the maximum height attained by a body projected with a velocit...
Text Solution
|
- A block is dragged on a smooth plane with the help of a rope which mov...
Text Solution
|
- Two satellites S(1) and S(2) are revolving round a planet in coplanar ...
Text Solution
|
- A body of mass 4 kg moving with velocity 12 m//s collides with another...
Text Solution
|
- The Coefficient of cubical expansion of mercury is 0.0018//^(@)C and t...
Text Solution
|
- A particle of mass m is moving in a circular path of constant radius r...
Text Solution
|
- A body of mass 5xx10^(-3) kg is launched upon a rough inclined plane m...
Text Solution
|
- A boy is pulshing a ring of mass 3 kg and radius 0.6 m with a stick a...
Text Solution
|
- A body of mass m is released from a height h to a scale pan hung from ...
Text Solution
|
- In an experiment to measure the height of bridge by droping stone into...
Text Solution
|
- A person of weight 70 kg wants to loose 7 kg by going up and dwon 12 m...
Text Solution
|
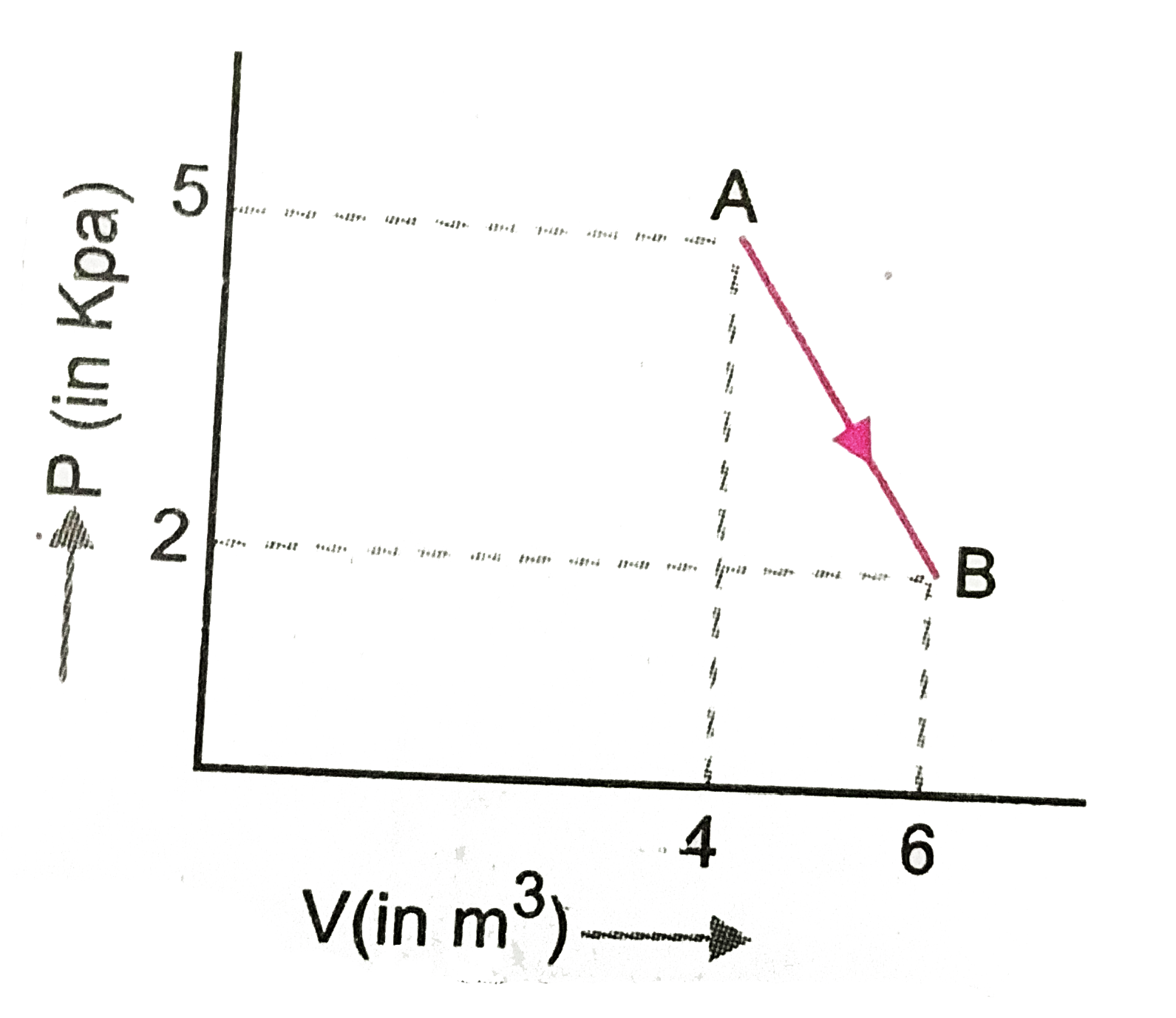
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- Assertion For looping a verticla loop of radius, r the minimum velocit...
Text Solution
|
- Assertion A spring of force constatn k is cut in to two piece having l...
Text Solution
|
- Assertion The total kinetic energy of a rolling solid sphere is the s...
Text Solution
|
- Assertion It is hotter over the top of a fire than at the same distanc...
Text Solution
|
- Assertion: When theta = 45^(@) or 135^(@), the value of R remains the ...
Text Solution
|
- Assertion In adiabatic expansion the product of p and V always decrese...
Text Solution
|
- Assertion : The molecules of a monatomic gas has three degrees freedom...
Text Solution
|
- Assertion Molar heat capacity cannot be defined for isothermal process...
Text Solution
|