A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY ENGLISH|Exercise Check point 14.2|20 VideosTHERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY ENGLISH|Exercise Check point 14.3|20 VideosTHERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY ENGLISH|Exercise Medical entrance gallary|30 VideosSUPERPOSITION OF WAVES
DC PANDEY ENGLISH|Exercise Level 2 Subjective|8 VideosTHERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES
DC PANDEY ENGLISH|Exercise Level 2 Subjective|9 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES-Check point 14.1
- A device used to measure very high temperature is
Text Solution
|
- On which of the following scales of temperature, the temperature is ne...
Text Solution
|
- A difference of temperature of 25^(@)C is equivalent to a difference o...
Text Solution
|
- The absolute zero temperature in Fahrenheit scale is
Text Solution
|
- The freezing point on a thermometer is marked as -20^(@) and the boili...
Text Solution
|
- At what temperature the Fahrenheit and Celsius scales of temperature g...
Text Solution
|
- A faulty thermometer has its fixed points marded 5 and 95. if the temp...
Text Solution
|
- The temperature of a body on Kelvin scale is found to be x K . When it...
Text Solution
|
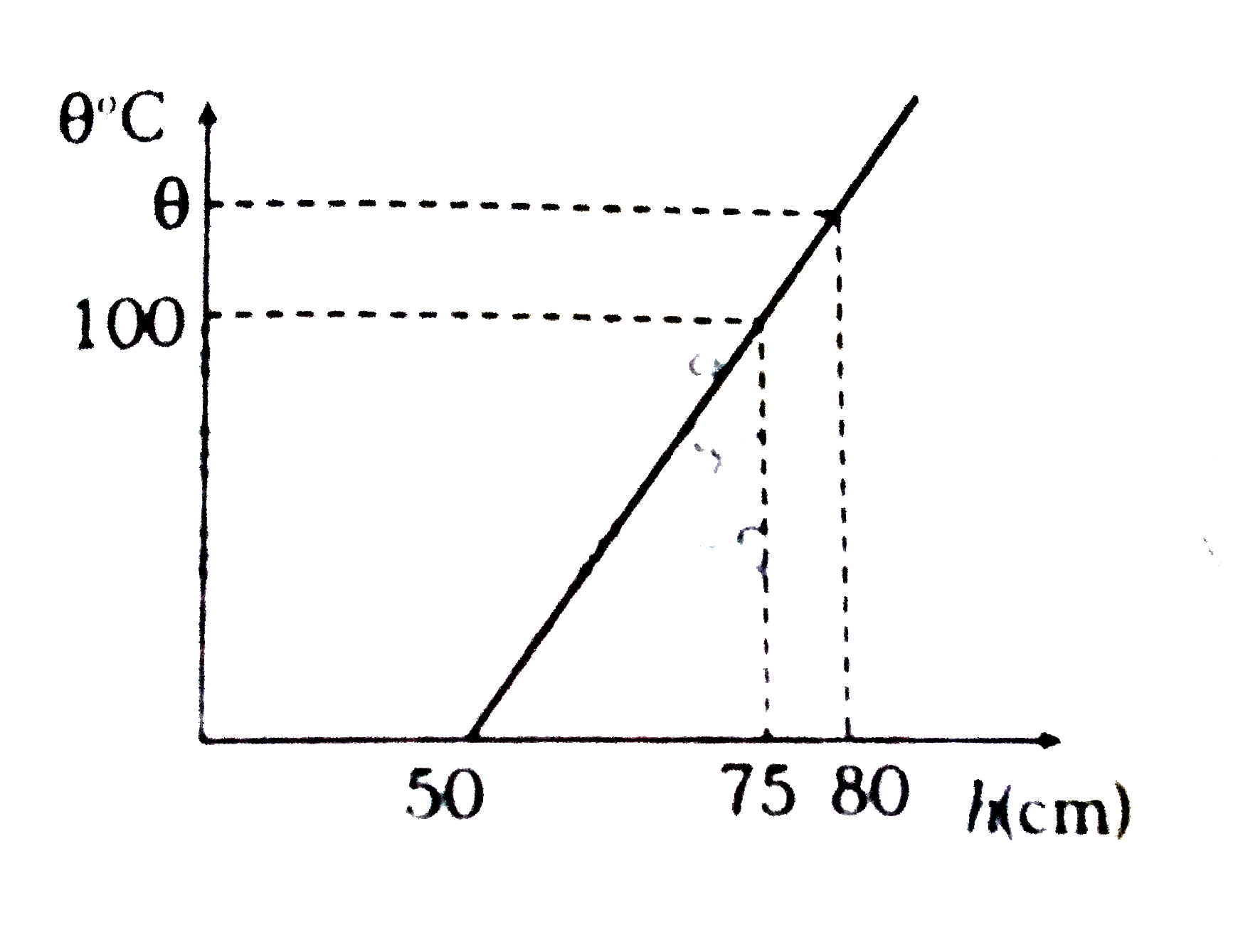
- The readings of a thermometer at 0^(@)C and 100^(@)C are 50 cm and 75 ...
Text Solution
|
- The readings of a bath on Celsius and Fahrenheit thermometers are in t...
Text Solution
|