A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY ENGLISH|Exercise A Tacking it together|55 VideosTHERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY ENGLISH|Exercise B medical entrance special format questions|14 VideosTHERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES
DC PANDEY ENGLISH|Exercise Check point 14.3|20 VideosSUPERPOSITION OF WAVES
DC PANDEY ENGLISH|Exercise Level 2 Subjective|8 VideosTHERMOMETRY,THERMAL EXPANSION & KINETIC THEORY OF GASES
DC PANDEY ENGLISH|Exercise Level 2 Subjective|9 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-THERMOMETRY THERMAL EXPANSION AND KINETIC THEORY OF GASES-Check point 14.4
- The mean kinetic energy of one mole of gas per degree of
Text Solution
|
- The average translational kinetic energy of O(2) (molar mass 32) molec...
Text Solution
|
- A perfect gas at 27^(@)C is heated at constant pressure so as to tripl...
Text Solution
|
- 16 gram of oxygen, 14 gram of nitrogen and 11 gram of carbon dioxide a...
Text Solution
|
- A balloon is filled at 27^(@)C and 1 atm pressure by 500 m^(3) He. At-...
Text Solution
|
- Aperfect gas at 27^(@) C is heated at constant pressure soas to duuble...
Text Solution
|
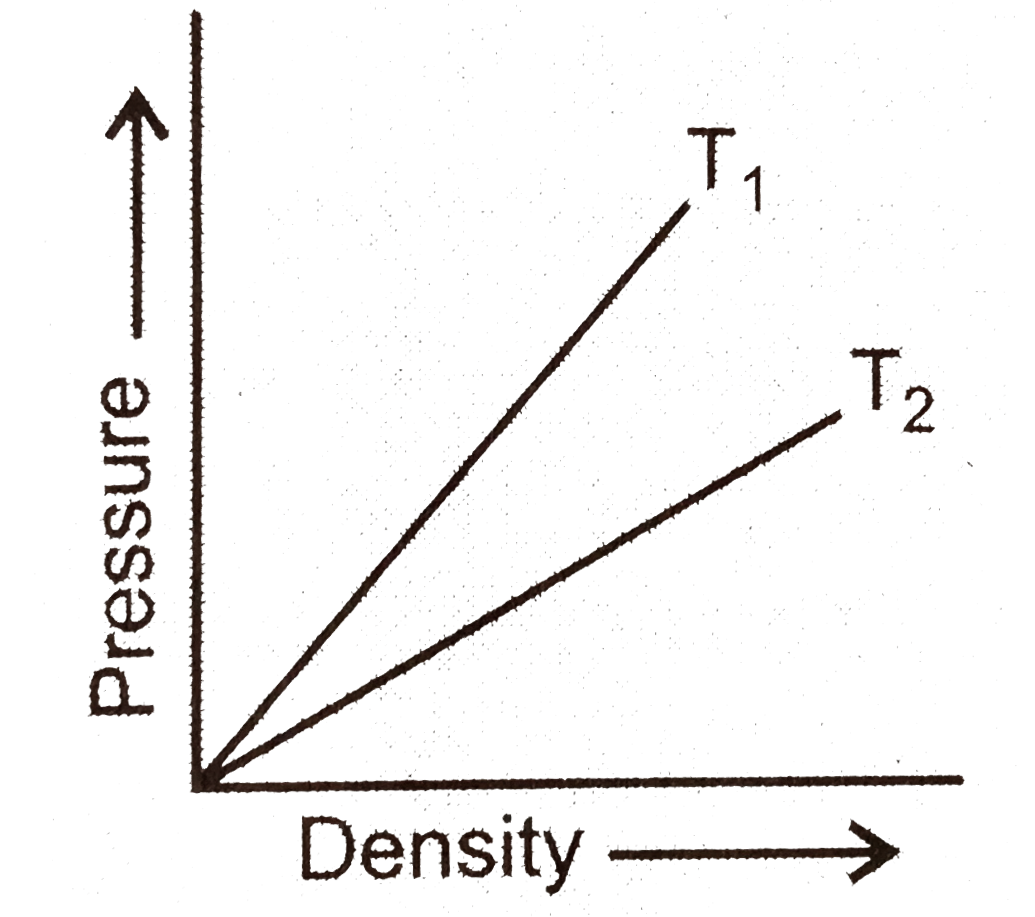
- Figure shows graphs of pressure versus density for an ideal gas at two...
Text Solution
|
- From the p-T graph what conclusion can be drawn?
Text Solution
|
- A cylinder containe 20 kg of N(2) gas (M= 28 kg K^(-1) mol^(-1)) at a ...
Text Solution
|
- Two different isotherms representing the relationship between pressure...
Text Solution
|
- A gas is found to obey the law P^(2)V = constant. The initial temperat...
Text Solution
|
- A gas has volume V and pressure p. The total translational kinetic ene...
Text Solution
|
- A vessel contains a mixture of one mole of Oxygen and two moles of Nit...
Text Solution
|
- Two monoatomic gases are at absolute temperatures 300 K and 350 K res...
Text Solution
|
- At 27^(@)C temperature, the kinetic energy of an ideal gas is E(1^.) I...
Text Solution
|
- Temperature remaining constant, the pressure of gas is decreased by 20...
Text Solution
|
- One litre of an ideal gas st 27^(@)C is heated at a constant pressure ...
Text Solution
|
- In the given (V-T) diagram, what is the relation between pressure P(1)...
Text Solution
|
- The gas in a vessel is subjected to a pressure of 20 atmosphere at a t...
Text Solution
|
- The graph which represents the variation of mean kinetic energy of mol...
Text Solution
|
 .
.