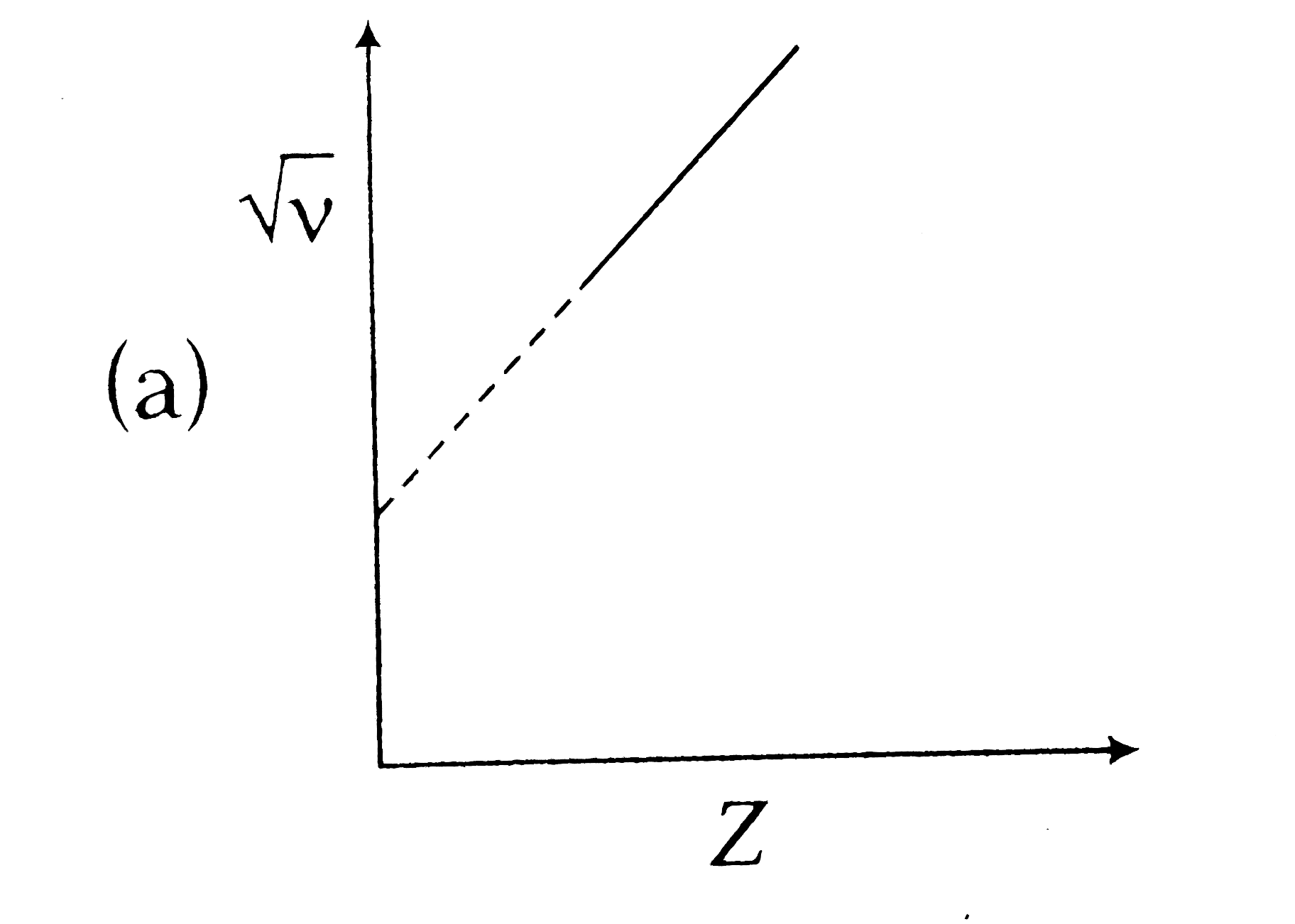
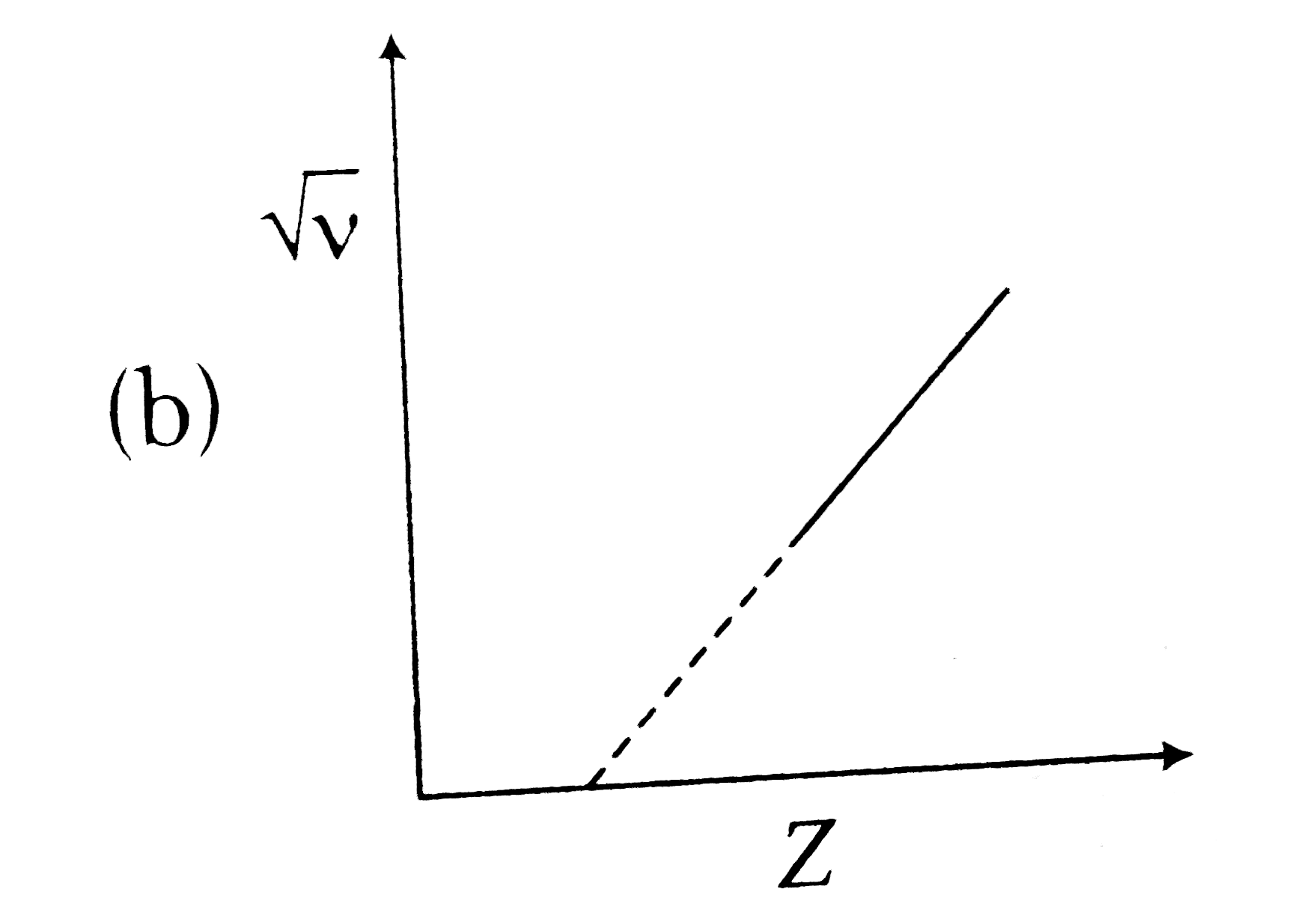
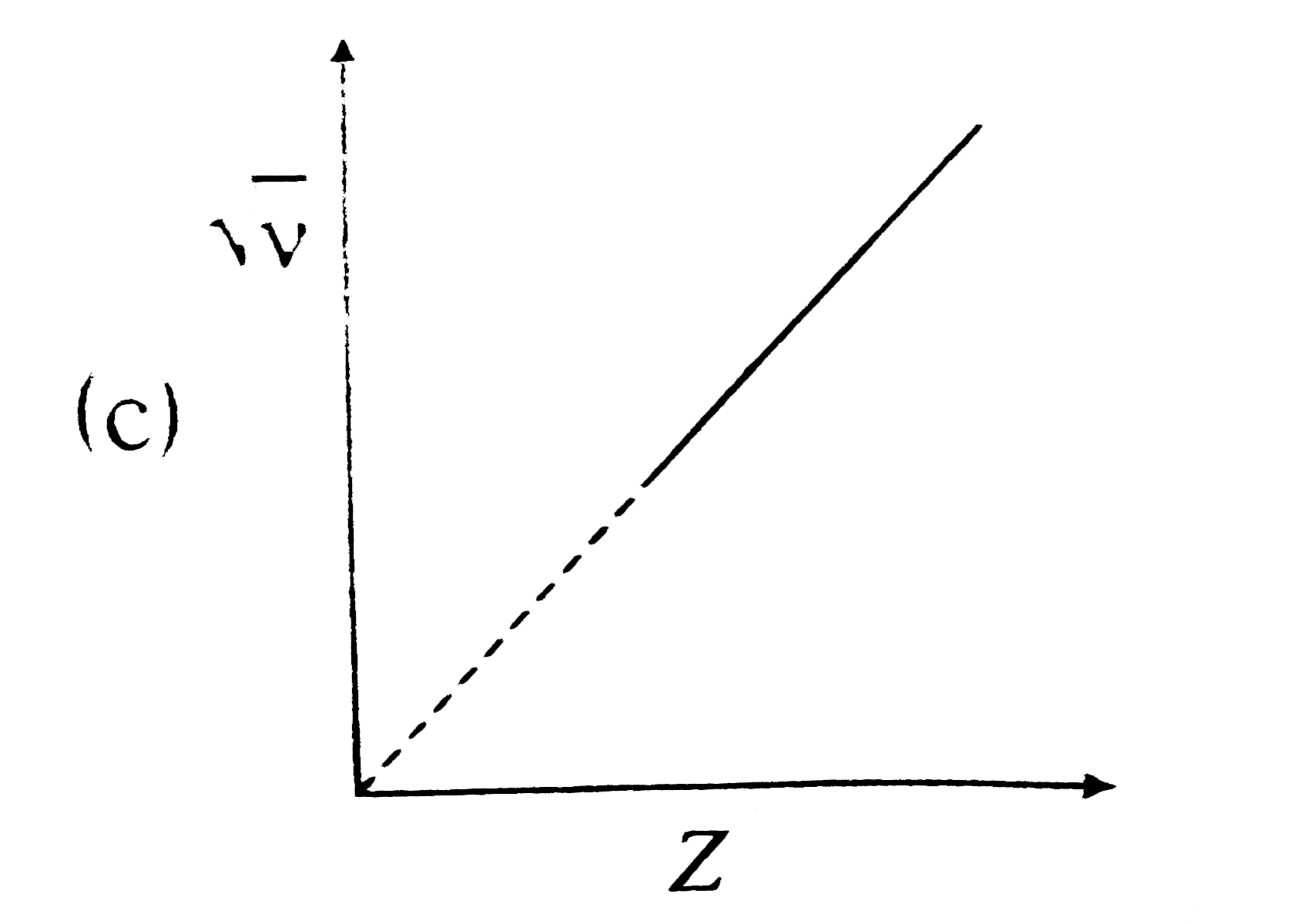
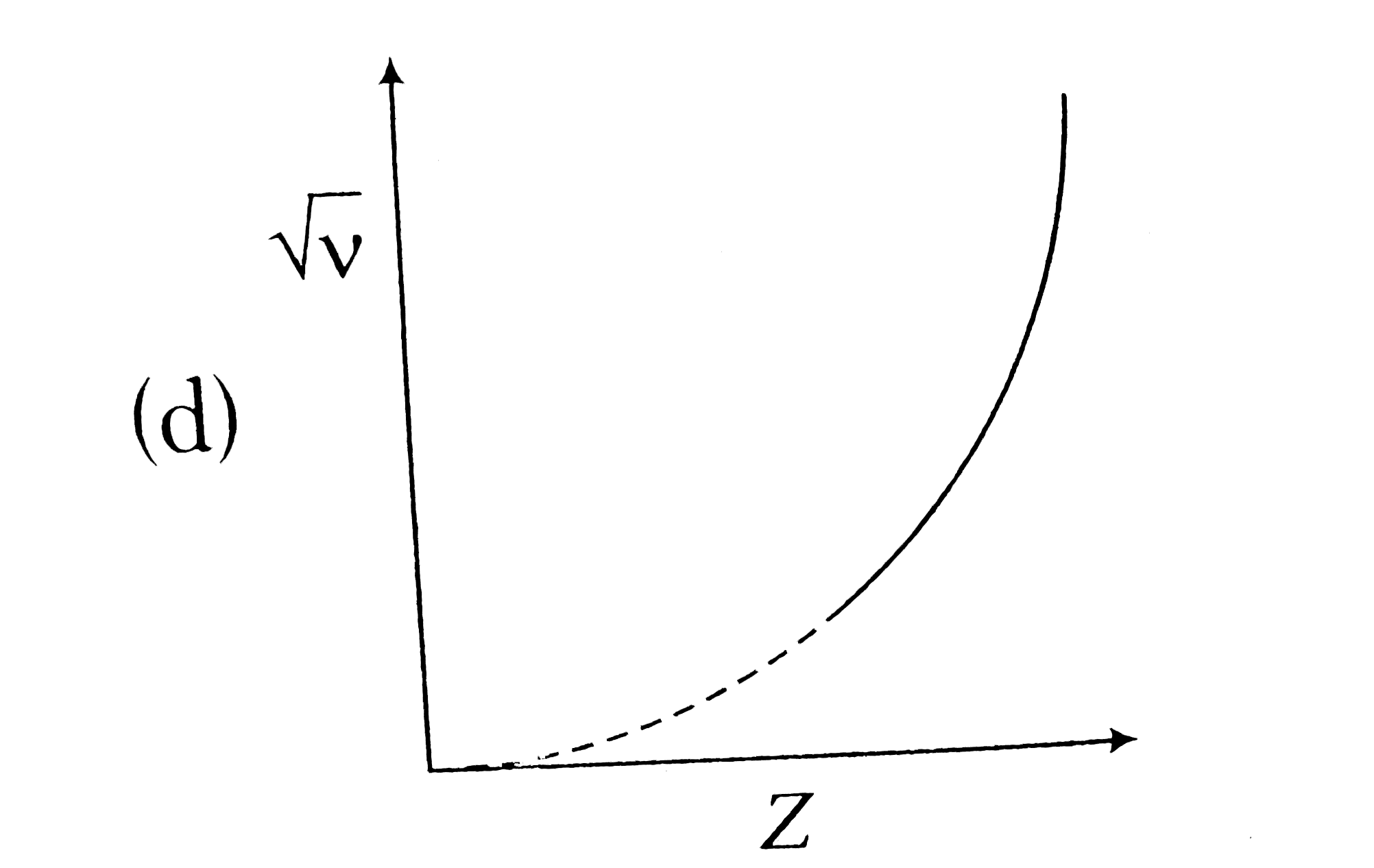
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ATOMS
DC PANDEY ENGLISH|Exercise ASSERTION AND REASON|10 VideosATOMS
DC PANDEY ENGLISH|Exercise MATCH THE COLUMNS|4 VideosATOMS
DC PANDEY ENGLISH|Exercise Check point 12.3|15 VideosALTERNATING CURRENT
DC PANDEY ENGLISH|Exercise JEE MAIN|62 VideosCAPACITORS
DC PANDEY ENGLISH|Exercise OBJECTIVE_TYPE|1 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-ATOMS-Taking it together
- The longest wavelength that can be analysed by a sodium chloride cryst...
Text Solution
|
- The wavelengths of Kalpha X-rays from lead isotopes Pb^(204) , Pb^(20...
Text Solution
|
- The graph between the square root of the frequency of a specific line ...
Text Solution
|
- The ratio between acceleration of the electron in singlely ionized hel...
Text Solution
|
- Magnetic moment of an electron in nth orbit of hydrogen atom is
Text Solution
|
- A sphere of work function phi=4.6 eV is suspended in a vacuum number b...
Text Solution
|
- The wavelength of radiation emitted is lambda(0) when an electron jump...
Text Solution
|
- Hard X-ray for the study of fractures in bones should have a minimum w...
Text Solution
|
- An electron with kinetic energy 5eV is incident on a hydrogen atom in ...
Text Solution
|
- An electron makes a transition from orbit n=4 to the orbit n=2 of a hy...
Text Solution
|
- The minimum energy to ionize an atom is the energy required to
Text Solution
|
- Let v(1) be the frequency of the series limit of the Lyman series, v(2...
Text Solution
|
- The shortest wavelength of the Brackett series of a hydrogen-like ato...
Text Solution
|
- When an alpha-particle of mass m moving with velocity v bombards on he...
Text Solution
|
- The energy of an electron in excited hydrogen atom is -3.4 eV . Then, ...
Text Solution
|
- The shortest wavelength which can be obtained in hydrogen spectrum (R=...
Text Solution
|
- The wavelength of th first spectral line of sodium 5896 Å . The fisrt ...
Text Solution
|
- The shortest wavelength in Lyman series is 91.2 nm. The longest wavele...
Text Solution
|
- Energy of 24.6 eV is required to remove one of the electron from a neu...
Text Solution
|
- If the radius of firs Bohr's orbit is x, then de-Broglie wavelenght of...
Text Solution
|