Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-THE SOLID STATE-QUESTION BANK
- A cubic unit cell is characterised by a=b=c and alpha=beta=gamma=90^@ ...
Text Solution
|
- A cubic unit cell is characterised by a=b=c and alpha=beta=gamma=90^@ ...
Text Solution
|
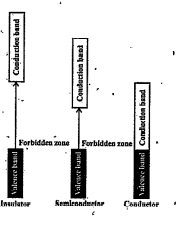
- Solids can be classified into three types on the basis of their electr...
Text Solution
|
- Solids can be classified into three types on the basis of their electr...
Text Solution
|
- Schottky and frenkel defects are stoichiometric defects. Write any tw...
Text Solution
|
- Schottky and frenkel defects are stoichiometric defects. When pure NaC...
Text Solution
|
- NaCl has fcc structure. Calculate the number of NaCl unit cell of NaCl
Text Solution
|
- Calculate the density of NaCl, if the edge length of NaCl unit cell is...
Text Solution
|
- Unit cell can be broadly classified into 2 categories-primitive and ce...
Text Solution
|
- Unit cell can be broadly classified into 2 categories-primitive and ce...
Text Solution
|
- The unit cell dimension of a particular crystal system is a=b=c, alpha...
Text Solution
|
- Give one example for the above crystal system.
Text Solution
|
- Every substance has some magnetic properties associated with it. How w...
Text Solution
|
- Every substance has some magnetic properties associated with it. How w...
Text Solution
|
- A compound is formed by two elements P and Q . Atoms Q (as anions) mak...
Text Solution
|
- Crystalline solids are 'anisotropic'. What is anisotropy?
Text Solution
|
- Copper crystals have fcc unit cells: Compute the number of atoms per...
Text Solution
|
- Copper crystals have fcc unit cells: Calculate the mass of a unit ce...
Text Solution
|
- Unit cell can be divided into two categories primitive and centred uni...
Text Solution
|
- Unit cell can be divided into two categories primitive and centred uni...
Text Solution
|