Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-SOLUTIONS-QUESTION BANK
- Colligative properties are properties of solutions which depend on the...
Text Solution
|
- Colligative properties are properties of solutions which depend on the...
Text Solution
|
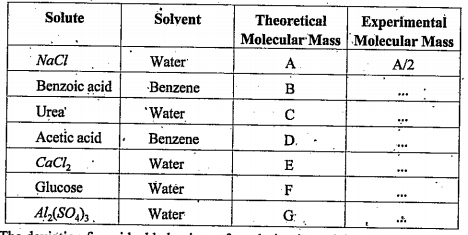
- Mr. Raju has determined the molecular masses of different solutes in d...
Text Solution
|
- What is the significant of van't Hoff factor ?
Text Solution
|
- Elevation of boiling point is a colligative property. What is colligat...
Text Solution
|
- Colligative properties can be used to determine the molecular mass of...
Text Solution
|
- For intravenous injections, only solutions with freezing point depress...
Text Solution
|
- Relative lowering of vapour pressure, elevation of boiling point, depr...
Text Solution
|
- An aqueous solution solution of a non-volatile solute boils at 373.053...
Text Solution
|
- Vapour pressure of a solution is different from that of pure solvent. ...
Text Solution
|
- Vapour pressure of chloroform (CHCl3) and dichloro methane (CH2Cl2) at...
Text Solution
|
- Colligative properties are properties of solution which depends on the...
Text Solution
|
- Colligative properties are properties of solution which depends on the...
Text Solution
|
- Elevation of boiling point is a colligative property. What is colligat...
Text Solution
|
- The boiling point of benzene is 353.23K. When 1.80g of a non-volatile ...
Text Solution
|
- Liquid solutions can be classified as ideal and non-ideal solution on ...
Text Solution
|
- Liquid solutions can be classified as ideal and non-ideal solution on ...
Text Solution
|
- Liquid solutions can be classified as ideal and non-ideal solution on ...
Text Solution
|
- Liquid solutions can be classified as ideal and non-ideal solution on ...
Text Solution
|
- Osmotic pressure is a colligative property and it is proportional to t...
Text Solution
|
 .
.