Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-SOLUTIONS-QUESTION BANK
- State Henry's law.
Text Solution
|
- Write any two applications of Henry's law.
Text Solution
|
- 1000 cm^3 of an aqueous solution of a protein contain 1.26gm of the pr...
Text Solution
|
- The mole fraction of water in a mixture containing equal number of mol...
Text Solution
|
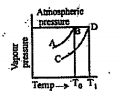
- The following are the vapour pressure curves answer the following ques...
Text Solution
|
- The following are the vapour pressure curves answer the following ques...
Text Solution
|
- Mixing of acetone and chloroform occurs with reduction in volume and i...
Text Solution
|
- Rectified spirit is a mixture of alcohol and water which behaves like ...
Text Solution
|
- Graphical representation of solution and a solvent for boiling of 0.1 ...
Text Solution
|
- Will elevation is boiling point same for a 0.1m NaCl solution and 0.1 ...
Text Solution
|
- Solution exhibit certain colligative properties such as elevation in b...
Text Solution
|
- solution exhibit certain colligative properties such as elevation in b...
Text Solution
|
- The value of molecular mass determined by colligative properties are s...
Text Solution
|
- The value of molecular mass determined by colligative properties are s...
Text Solution
|
- Out of the following solutions which has the lowest freezing point ? G...
Text Solution
|
- Explain why the molecular masses of some substances determined with th...
Text Solution
|
- To get hard boiled eggs, common salt is added to water during boiling ...
Text Solution
|
- Which is the colligative property, osmosis or osmotic pressure ? Sugge...
Text Solution
|
- Account for the following NaCl is used to remove ice from road in cold...
Text Solution
|
- Account for the following Ethylene glycol is added to radiator in auto...
Text Solution
|