Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-ELECTROCHEMISTRY-QUESTION BANK
- Kohlrausch's law helps to determine the degree of dissociation of a we...
Text Solution
|
- Kohlrausch's law helps to determine the degree of dissociation of a we...
Text Solution
|
- Represent the electrochemical cells corresponding to the following cel...
Text Solution
|
- Represent the electrochemical cells corresponding to the following cel...
Text Solution
|
- What will be the standard EMF of the cell with reaction (i) if E(red)^...
Text Solution
|
- What is resistivity?Give its unit.
Text Solution
|
- How is specific conductivity related to resistivity?
Text Solution
|
- The limiting molar conductivity of an electrolyte is obtained by addin...
Text Solution
|
- Calculate the molar conductivity of 1M solution of sulphuric acid if i...
Text Solution
|
- It is not possible to determine the molar conductivity of weak electro...
Text Solution
|
- It is not possible to determine the molar conductivity of weak electro...
Text Solution
|
- It is not possible to determine the moral conductivity of weak electro...
Text Solution
|
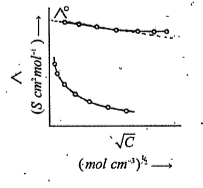
- The following is a plot of molar conductivity of electrolytesA and B a...
Text Solution
|
- The following is a plot of molar conductivity of electrolytesA and B a...
Text Solution
|
- The following is a plot of molar conductivity of electrolytesA and B a...
Text Solution
|
- Dry cell is a compact version of Leclanche cell and provides voltage b...
Text Solution
|
- Dry cell is a compact version of Leclanche cell and provides voltage b...
Text Solution
|
- Dry cell is a compact version of Leclanche cell and provides voltage b...
Text Solution
|
- KCL cannot be used in salt bridge inZn//Zn^(+2)////Ag^(+)//Ag.Give rea...
Text Solution
|
- What happens to pH when aq NaCl is subjected to electrolysis?
Text Solution
|