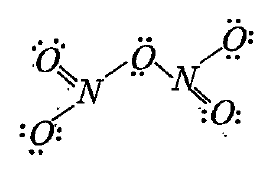
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-P-BLOCK ELEMENTS-QUESTION BANK
- The principle goal of chemical synthesis is to maximize the conversion...
Text Solution
|
- How does ammonia react with a solution of Cu^(2+) ?
Text Solution
|
- What is the covalence of nitrogen in N2O5?
Text Solution
|
- Bond angle in is PH4^+ higher than that in PH3
Text Solution
|
- What happens when white phosphorus is heated with NaOH solution in an...
Text Solution
|
- What happens when PCl5 is heated?
Text Solution
|
- Write balanced equation for the hydrolytic reaction of PCl5 with heav...
Text Solution
|
- What is the basicity of H3PO4?
Text Solution
|
- What happens when H3PO3 is heated?
Text Solution
|
- List the important sources of sulphur
Text Solution
|
- Write the order thermal stability of the hydrides of group-16 elements
Text Solution
|
- H2S is a gas at ordinary condition, while H2O is liquid. Account for t...
Text Solution
|
- Which of the following does not react with O2 directly Zn, Ti,Pt, Fe?
Text Solution
|
- Complete the following reactions? C2H4+O2rarr
Text Solution
|
- Complete the following reactions? '4Al+3O2
Text Solution
|
- Why does ozone act as a powerful oxidising agent?
Text Solution
|
- Suggests method for the quantitative estimation of ozone (O3).
Text Solution
|
- What happens when SO2 is passed through an aqueous solution of Fe (III...
Text Solution
|
- Comment on the nature of two S-O bonds in SO2 molecule. Are the two S:...
Text Solution
|
- How is the presence of SO2 detected?
Text Solution
|