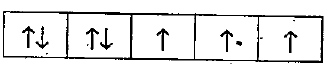
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-D AND F - BLOCK ELEMENTS-QUESTION BANK
- Why is the highest oxidation state of a metal exhibited in its oxide o...
Text Solution
|
- Which is a stronger oxidising agent Cr^2+orFe^(2+) Why?
Text Solution
|
- Calculate the ‘spin only’ magnetic moment of M^(2+) (aq) ion (Atomic ...
Text Solution
|
- Explain why Cu^+ ion is not stable in aqueous solution?
Text Solution
|
- Actinoid contraction is greater from element to element than lanthanoi...
Text Solution
|
- Transition elements are d- block elements Write any four characteristi...
Text Solution
|
- Lanthanides and actinoids are f-block elements What is the common oxi...
Text Solution
|
- Lanthanoids and actinoids are f-block elements Name the lanthanoid ha...
Text Solution
|
- Lanthanoids and actinoids are f-block elements It is difficult to sep...
Text Solution
|
- Transition metals are widely used as catalysts in industrial processes...
Text Solution
|
- Transition metals are widely used as catalysts in industrial processes...
Text Solution
|
- Transition metals are widely used as catalysts in industrial processes...
Text Solution
|
- Atomic sizes generally increase as we come down a group of the periodi...
Text Solution
|
- E^@ (standard electrode potential) values generally become less negati...
Text Solution
|
- Transition elements are d-block elements, with some exceptions. Usuall...
Text Solution
|
- Transition elements are d-block elements, with some exceptions. Usuall...
Text Solution
|
- Which of the 3d series of elements exhibits the largest number of oxid...
Text Solution
|
- Transition elements are d-block elements, with some exceptions. Usuall...
Text Solution
|
- Describe the method of preparation of potassium dichromate from chromi...
Text Solution
|
- Write any one consequence of lanthanoid contraction.
Text Solution
|