Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-D AND F - BLOCK ELEMENTS-QUESTION BANK
- Transition elements are d- block elements Write any four characteristi...
Text Solution
|
- Transition elements are d - block elements and inner transition elemen...
Text Solution
|
- Transition elements are d - block elements and inner transition elemen...
Text Solution
|
- Transition elements are d - block elements and inner transition elemen...
Text Solution
|
- Transition elements are d- block elements Write any four characteristi...
Text Solution
|
- transition elements are 'd' block elements. Cr^(2+) and Mn^(3+) have d...
Text Solution
|
- Which of the following is not a lanthanoid element?
Text Solution
|
- Zr and Hf are having similar chemical properties. This is due to.
Text Solution
|
- Magnetic moments arise due to the presence of unpaired electrons’ Calc...
Text Solution
|
- Transition metal ions are generally coloured. Why?
Text Solution
|
- A transition element is defined as one which has incompletely filled d...
Text Solution
|
- Silver atom has completely-filled d-orbital (4d^105 s^1) in its groun...
Text Solution
|
- Why are d block elements called transition elements?
Text Solution
|
- Transition elements generally have high enthalpy of atomisation and th...
Text Solution
|
- In the 3d series, the .enthalpy of atomisation of zinc is the lowest (...
Text Solution
|
- Name a transition element which does not exhibit variable oxidation st...
Text Solution
|
- Transition elements generally have high enthalpy of atomisation and th...
Text Solution
|
- Rationalise the following observations Scandium salts are white/colo...
Text Solution
|
- Atomic sizes generally increase as we come down a group of the periodi...
Text Solution
|
- Rationalise the following observations Zinc salts are colourless, Cu^(...
Text Solution
|
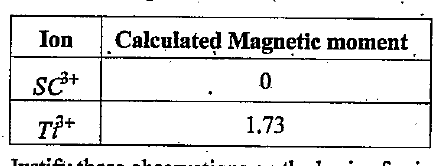 Justify these observations on the basis of spin only formula.
Justify these observations on the basis of spin only formula.