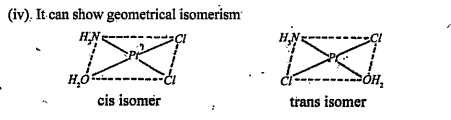
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-COORDINATION COMPOUNDS-QUESTION BANK
- Indicate the type of isomerism exhibited by the following complexes an...
Text Solution
|
- Indicate the type of isomerism exhibited by the following complexes an...
Text Solution
|
- Indicate the type of isomerism exhibited by the following complexe and...
Text Solution
|
- Give evidence that [Co[NH3)5Cl]SO4 and [Co(NH3)5SO4]Cl are ionisation ...
Text Solution
|
- Explain on the basis of V.B, theory that [Ni(CN)4]^(2-) ion with squar...
Text Solution
|
- [NiCl4]^(2-) is paramagnetic while Ni(CO)4 is diamagnetic though both ...
Text Solution
|
- [Fe(H2O)6]^(3+) is strongly paramagnetic whereas {Fe(CN)6]^(3-) is wea...
Text Solution
|
- [Co(NH3)6]^3+ is an inner orbital complex whereas [Ni(NH3)6]^(2+) is a...
Text Solution
|
- Predict the number of unpaired electrons in the squar planar [Pt(CN)4]...
Text Solution
|
- The hexaaquamanganese(II)ion contains five unpaired electrons,while th...
Text Solution
|
- Calculate the overall complex dissociation equillibrium constant for t...
Text Solution
|
- [Cr(NH3)4Cl2]Br is a coordination compound. Identify the central metal...
Text Solution
|
- [Cr(NH3)4Cl2]Br is a coordination compound.Name the ligands present in...
Text Solution
|
- [Cr(NH3)4Cl2]Br is a coordination compound. What is its coordination n...
Text Solution
|
- [Cr(NH3)4Cl2]Br is a coordination compound. Write its IUPAC name.
Text Solution
|
- [Cr(NH3)4Cl2]Br is a coordination compound.Write the ionisation isomer...
Text Solution
|
- When CuSO4 is mixed with excess of NH3, a deep blue coloured solution ...
Text Solution
|
- When CuSO4 is mixed with excess of NH3, a deep blue coloured solution ...
Text Solution
|
- When CuSO4 is mixed with excess of NH3, a deep blue coloured solution ...
Text Solution
|
- When CuSO4 is mixed with excess of NH3, a deep blue coloured solution ...
Text Solution
|