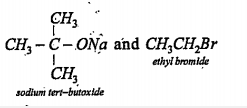Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-ALCOHOLS, PHENOLS AND ETHERS-QUESTION BANK
- How will you prepare the following compounds using Grignard reagent? ...
Text Solution
|
- Primary,secondary and tertiary alcohols can be distinguished by Lucas ...
Text Solution
|
- Write the correct pair of reactants for the preparation of t-butyl eth...
Text Solution
|
- Write the name or formula of the following: A simple ether
Text Solution
|
- Write the name or formula of the following: A mixed ether
Text Solution
|
- Write the name or formula of the following: A dihydric alcohol
Text Solution
|
- Write the name or formula of the following: A trihydric alcohol
Text Solution
|
- Phenol on treatment with Br2 in CS2 at low temperature gives two isome...
Text Solution
|
- Alocohols are compounds with general formula R-OH Alcohols are solub...
Text Solution
|
- Alocohols are compounds with general formula R-OH Explain a method f...
Text Solution
|
- Alocohols are compounds with general formula R-OH How will you conve...
Text Solution
|
- Write a test to distinguish between phenol and alcohol
Text Solution
|
- Write suitable reagent used for the following conversions: CH3-CH2-Cl...
Text Solution
|
- Write suitable reagent used for the following conversion: CH3-CH2-OH ...
Text Solution
|
- Write suitable reagent or reagents usrd for the following conversions:
Text Solution
|
- Complete the following:
Text Solution
|
- Explain the following : Esterification
Text Solution
|
- Write the correct pair of reactants for the preparation of t-butyl eth...
Text Solution
|
- Phenols are more acidic than alcohols. What is the product obtained ...
Text Solution
|
- Methanol and ethanol are two commercially important alcohols. Write on...
Text Solution
|