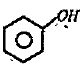
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-ALDEHYDE, KETONES AND CARBOXYLIC ACIDS-QUESTION BANK
- How will you make the following conversions? Toluene to benzoic acid.
Text Solution
|
- Following are a group of compounds showing acidic behaviour: Give...
Text Solution
|
- Following are a group of compounds showing acidic behaviour:does not c...
Text Solution
|
- Following are a group of compounds showing acidic behaviour: Phenols a...
Text Solution
|
- Following are a group of compounds showing acidic behaviour: Formic ac...
Text Solution
|
- is an aromatic acid. What is it IUPAC name?
Text Solution
|
- Explain the conversion of the 3,4-dinitrobenzoic acid to the following...
Text Solution
|
- Explain the conversion of the above acid to the following.
Text Solution
|
- write the conversion of the 3,4-dinirtobenzoic acid to the following.
Text Solution
|
- Aldehydes resemble ketones in many respects. Give the reason for their...
Text Solution
|
- Aldehydes resemble ketones in many respects. Give a reaction in which ...
Text Solution
|
- Write a test to distinguish between aldehydes and ketones.
Text Solution
|
- Aldehydes resemble ketones in many respects. What is Cannizzaro reacti...
Text Solution
|
- Which named reaction is used to reduce CH3COCl to CH3CHO?
Text Solution
|
- Aldehydes and ketones undergo reactions due to the presence of alpha-h...
Text Solution
|
- How will you bring about the above reaction?
Text Solution
|
- CH2ClCOOH is a stronger acid than CH3COOH. Why?
Text Solution
|
- How will you convert CH3COOH to CH2ClCOOH?
Text Solution
|
- Complete the following. Write down the structures of A, B and C. (i) C...
Text Solution
|
- Complete the following. Write down the structure of B. (i) CH3-CH2-CO...
Text Solution
|