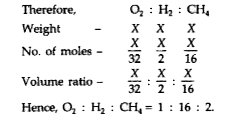Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Equal masses of oxygen, hydrogen and methane are taken in a container ...
Text Solution
|
- Equal masses of H(2) , O(2) and methane have been taken in a container...
Text Solution
|
- Equal masses of oxygen, hydrogen and methane are taken in a container ...
Text Solution
|
- Equal masses of oxygen, hydrogen and methane are taken in identical co...
Text Solution
|
- Equal masses of H(2), O(2) and methane have been taken in a container ...
Text Solution
|
- ऑक्सीजन, हाइड्रोजन तथा मेथेन के समान द्रव्यमान, समान परिस्थितियों में ...
Text Solution
|
- Equal masses of oxygen hydrogen and methane are taken in a container u...
Text Solution
|
- Equal masses of H(2),O(2) and methane have been taken in a container o...
Text Solution
|
- Equal masses of H(2), O(2) and methane have been taken in a container ...
Text Solution
|