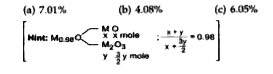A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Experimentally it was found that a metal oxide has formula M(0.98) O. ...
Text Solution
|
- Experimentally it is was found that a metal oxide has formula M(0.95)O...
Text Solution
|
- Experimentally it was found that a metal oxide in formula M(0.98)O . M...
Text Solution
|
- Experimentally it was found that a metal oxide has formula M(0.98)O. M...
Text Solution
|
- प्रयोग के आधार पर एक धातु ऑक्साइड का सूत्र M(0.98)O पाया गया । यदि धात...
Text Solution
|
- प्रयोगिक रूप से ज्ञात किया गया कि किसी धातु ऑक्साइड का सूत्र M(0.98)O ...
Text Solution
|
- Experimentally it was found that a metal oxide has dormula M(0.98)O. M...
Text Solution
|
- An oxide of a metal (M) contains 40% by mass of oxygen. Metal (M) has ...
Text Solution
|
- प्रयोग के आधार पर एक धातु ऑक्साइड का सूत्र M 0.98^0 पाया गया। यदि धातु...
Text Solution
|