Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-CLASSIFICATION TO ELEMENTS AND PERIODICITY IN PROPERTIES-QUESTION BANK
- Assing the position of the elements with outer electronic configuratio...
Text Solution
|
- Assing the position of the elements with outer electronic configuratio...
Text Solution
|
- The first IE and second IE (Kj mol^(-1)) and daltaegH(kJ mol^(-1)) of...
Text Solution
|
- The first IE and second IE (Kj mol^(-1)) and daltaegH(kJ mol^(-1)) of...
Text Solution
|
- The first IE and second IE (Kj mol^(-1)) and daltaegH(kJ mol^(-1)) of...
Text Solution
|
- The first IE and second IE (Kj mol^(-1)) and daltaegH(kJ mol^(-1)) of...
Text Solution
|
- The first IE and second IE (Kj mol^(-1)) and daltaegH(kJ mol^(-1)) of...
Text Solution
|
- The first IE and second IE (Kj mol^(-1)) and daltaegH(kJ mol^(-1)) of...
Text Solution
|
- Predict the formulae of stable binary compounds that would be the form...
Text Solution
|
- Predict the formulae of stable binary compounds that would be the form...
Text Solution
|
- Predict the formulae of stable binary compounds that would be the form...
Text Solution
|
- Predict the formulae of stable binary compounds that would be the form...
Text Solution
|
- Predict the formulae of stable binary compounds that would be the form...
Text Solution
|
- Predict the formulae of stable binary compounds that would be the form...
Text Solution
|
- In the modern periodic table, the period indicates the value of
Text Solution
|
- The size of iso electronic species F-Ne and Na^+ is affected by
Text Solution
|
- Considering the elements B,Al,Mg and K, the correct order of their me...
Text Solution
|
- considering the elements F,Cl, O and N, the correct order their chemic...
Text Solution
|
- Account for the following: Ionisation enthalphy of nitrogen is greater...
Text Solution
|
- Account for the following: Atomic radius decreases from left to right ...
Text Solution
|
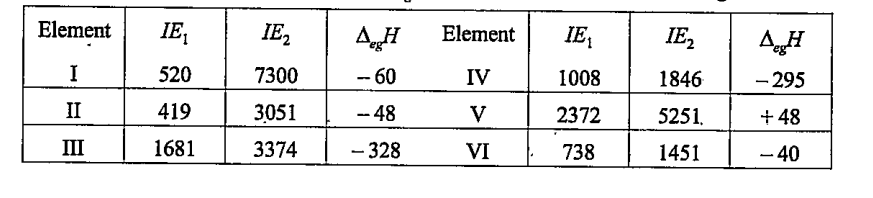 Which of the above is likely to be the least reactive element
Which of the above is likely to be the least reactive element