Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-QUESTION BANK
- Explain the struture of CO3^-2 ion in terms of resonance.
Text Solution
|
- Explain the structure of CO2 molecule.
Text Solution
|
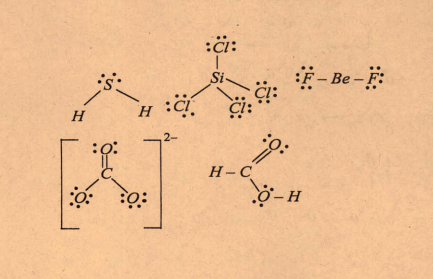
- Draw the Lewis structures for the following molecules and ions: H2S,...
Text Solution
|
- Disuss the shapes of the following molecules using VSEPR theory: BeCl2...
Text Solution
|
- Although geometries of NH3 and H2O molecules are distorted tetrahedral...
Text Solution
|
- How is bond strength related to bond order?
Text Solution
|
- Write the resonance structures of SO3, NO2 and NO3^-
Text Solution
|
- Although both CO2 and H2O are triatomic molecules.,the shape of H2O mo...
Text Solution
|
- Arrange the bonds in the order of increasing ionic character in the fo...
Text Solution
|
- Explain why BeH2 molecule has zero diple moment even though the Be-H b...
Text Solution
|
- Which out of NH3 and NF3 has higher dipole moment and why?
Text Solution
|
- Disuss the change in hybridisation of Al atom in the following reactio...
Text Solution
|
- Is there any change in the hybridisation of B and N atoms as a result ...
Text Solution
|
- Considering x- axis as the internuclear axis,which out of the followin...
Text Solution
|
- Which hybird orbitals are used by carbon atoms in the following molecu...
Text Solution
|
- Which hybird orbitals are used by carbon atoms in the following molecu...
Text Solution
|
- Which hybird orbitals are used by carbon atoms in the following molecu...
Text Solution
|
- Which hybird orbitals are used by carbon atoms in the following molecu...
Text Solution
|
- Which hybird orbitals are used by carbon atoms in the following molecu...
Text Solution
|
- What do you understand by bond pairs and lone pairs of electrons? Illu...
Text Solution
|