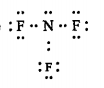Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-QUESTION BANK
- How is bond order related to bond length?
Text Solution
|
- Write the electronic configuration of an oxygen molecule and justify i...
Text Solution
|
- Only valence electrons of atoms take part in chemical combination. Dra...
Text Solution
|
- Define dipole moment.
Text Solution
|
- Based on M.O elecrtonic configuration, compare the magnetic property o...
Text Solution
|
- He2 cannot exist as stable molecule. Justify this statement on the bas...
Text Solution
|
- State Fajan's rule regarding the partial covalent character of an ioni...
Text Solution
|
- Which has higher boiling point: o-nitrophenol or p-nitophenol? Give re...
Text Solution
|
- Molecular orbitals are formed by the linear combination of atomic orbi...
Text Solution
|
- Explain sp^3d hybridization with a suitable example.
Text Solution
|
- The shapes of the molecules is based on the VSEPR theory. Give the sal...
Text Solution
|
- Draw the potential energy curve for the formation of a hydrogen molecu...
Text Solution
|
- One-half of the difference between the number of electrons in the bond...
Text Solution
|
- Calculate the bond order of dinitrogen(N2)
Text Solution
|
- Predict stability and magnetic property of N2 with reasons
Text Solution
|
- In order to explain the geometrical shapes of molecules, the concept o...
Text Solution
|
- In order to explain the geometrical shapes of molecules, the concept o...
Text Solution
|
- Explain sp^3 hybridisation taking methane (CH4) as an example
Text Solution
|
- Write the molecular orbital configuration of F2 molecule
Text Solution
|
- What is bond order?
Text Solution
|