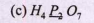Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-REDOX REACTIONS-QUESTION BANK
- Balance the following equation by the half reaction method. Fe^(2+)...
Text Solution
|
- Permanganate ion oxidises iodide to iodine in basic medium. Balance th...
Text Solution
|
- Assign o0XIdation number to the underlined elements in each of the fol...
Text Solution
|
- Assign oxidation number to the underlined elements in each of the foll...
Text Solution
|
- Assign o0XIdation number to the underlined elements in each of the fol...
Text Solution
|
- Assign oxidation number to the underlined elements in each of the foll...
Text Solution
|
- Assign oxidation number to the underlined element in the following spe...
Text Solution
|
- Assign o0XIdation number to the underlined elements in each of the fol...
Text Solution
|
- What are the o0XIdation number of the underlined elements in each of t...
Text Solution
|
- What are the o0XIdation number of the underlined elements in each of t...
Text Solution
|
- What are the o0XIdation number of the underlined elements in each of t...
Text Solution
|
- What are the o0XIdation number of the underlined elements in each of t...
Text Solution
|
- What are the oxidation number of the underlined elements in each of th...
Text Solution
|
- Justify the given reaction is redox reaction CuO (s) + H2 (g) toCu (...
Text Solution
|
- Justify the following reaction is redox reaction Fe2O3 (s) + 3CO (g...
Text Solution
|
- Justify the following reaction is redox reaction 4BCl3 (g) + 3LiAlH4...
Text Solution
|
- Justify the given reaction is redox reaction 2K (s) + F2 (g) to 2FK ...
Text Solution
|
- Justify the following reaction is redox reaction 4NH3(g) + 5O2 (g) t...
Text Solution
|
- Fluorine react with ice and results in the change
Text Solution
|
- Calculate the oxidation number of 's' in H2SO4 ?
Text Solution
|