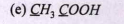Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A N EXCEL PUBLICATION-REDOX REACTIONS-QUESTION BANK
- What are the o0XIdation number of the underlined elements in each of t...
Text Solution
|
- What are the o0XIdation number of the underlined elements in each of t...
Text Solution
|
- What are the oxidation number of the underlined elements in each of th...
Text Solution
|
- Justify the given reaction is redox reaction CuO (s) + H2 (g) toCu (...
Text Solution
|
- Justify the following reaction is redox reaction Fe2O3 (s) + 3CO (g...
Text Solution
|
- Justify the following reaction is redox reaction 4BCl3 (g) + 3LiAlH4...
Text Solution
|
- Justify the given reaction is redox reaction 2K (s) + F2 (g) to 2FK ...
Text Solution
|
- Justify the following reaction is redox reaction 4NH3(g) + 5O2 (g) t...
Text Solution
|
- Fluorine react with ice and results in the change
Text Solution
|
- Calculate the oxidation number of 's' in H2SO4 ?
Text Solution
|
- Calculate the oxidation number of Cr in CrO5 .
Text Solution
|
- Calculate the oxidation number of N in NO3^- .
Text Solution
|
- Write formulas for the following compounds: a) Mercury (II) chloride
Text Solution
|
- Write the formula of the following compounds. Nickel Sulphate
Text Solution
|
- Write the formula of the following compound. Tin (IV) Oxide
Text Solution
|
- Write formula for the following compound: Thallium (I) sulphate
Text Solution
|
- Write formula for the following compound: Iron (III) Sulphate
Text Solution
|
- Write formula for the following compound: Chromium (III) oxide
Text Solution
|
- While SO2 and H2O2 act as oXIdising as well as reducing agents in reac...
Text Solution
|
- The compound AgF2 is unstable. However, if formed, the compound acts a...
Text Solution
|