A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
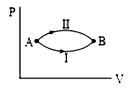
- A system goes from A to B via two processes I and II as shown in the f...
Text Solution
|
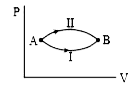
- Refer to let DeltaU(1) and DeltaU(2) be the changes in internal energy...
Text Solution
|
- Refer to let DeltaU(1) and DeltaU(2) be the changes in internal energy...
Text Solution
|
- A system goes from A and B via two processes. I and II as shown in fig...
Text Solution
|
- An ideal gas goes from State A to state B via three different process ...
Text Solution
|
- In given figure, let DeltaU(1) and DeltaU(2) be change in internal ene...
Text Solution
|
- Refer to figure. Let DeltaU(1) and DeltaU(2) be the changes in interna...
Text Solution
|
- Refer to figure. Let DeltaU(1) and DeltaU(2) be the changes in interna...
Text Solution
|
- A system goes from state A to B via two processes I and II, as shown i...
Text Solution
|