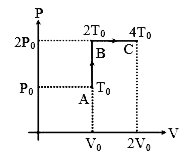
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
VIDYAMANDIR CLASSES (VMC MODULES)-QUIZ-PHYSICS
- In the following cyclic process is The above process in the P-T c...
Text Solution
|
- An ideal gas is expanded so that amount of heat given is equal to the ...
Text Solution
|
- In adiabatic process, the pressure is increased by 2//3%. If gamma=3/...
Text Solution
|
- A system goes from A and B via two processes. I and II as shown in fig...
Text Solution
|
- A diatomic ideal gas is heated at constant at constant volume until th...
Text Solution
|
- Four spring connect with mass as shown in figure. Find frequency of S....
Text Solution
|
- A uniform rod of length 2.0 m is suspended through an end and is set i...
Text Solution
|
- A uniform spring whose unstretched length is l has a force constant k....
Text Solution
|
- A clock with an iron pendulum keeps correct time at 20^(@)C. How much ...
Text Solution
|
- A particle executes SHM along a straight line so that its period is 12...
Text Solution
|
- A conducting shell of radius R carries charge -Q. A point charge +Q is...
Text Solution
|
- A uniformly charged and infinitely long line having a linear charge de...
Text Solution
|
- For a uniformly charged non conducting sphere of radius R which of fol...
Text Solution
|
- Two point charges q and –q are at positions (0,0,d) and (0,0, –d) resp...
Text Solution
|
- Two particles of mass m and 2m with charges 2q and q are placed in a u...
Text Solution
|
- A thin spherical conducting shell of radius R has a charge q. Another ...
Text Solution
|
- Two connectric spheres of radii R and r have similar charges with equa...
Text Solution
|
- An electric dipole of dipole moment 2xx10^(-10) coulomb is placed be...
Text Solution
|
- A rod of length 'l' is placed along x-axis. One of its ends is at the...
Text Solution
|
- The electirc potential at a point (x, y, z) is given by V = -x^(2)y ...
Text Solution
|