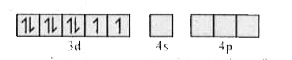Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 2)
SUNSTAR PUBLICATION|Exercise PART - D|28 VideosII PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 2)
SUNSTAR PUBLICATION|Exercise PART - B|9 VideosII PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 1)
SUNSTAR PUBLICATION|Exercise PART - D|25 VideosII PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 3)
SUNSTAR PUBLICATION|Exercise PART - D|30 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 2)-PART - C
- Complete the following equations: a) 2Cu(2)O+Cu(2)Srarr b) Zrl(4)o...
Text Solution
|
- Give reasons: i) Nitrogen exists as a diatomic molecule ii) Nitrog...
Text Solution
|
- Write the equations involved in the manufacture of sulphuric acid in c...
Text Solution
|
- Complete the following equations: i) Cl(2)+2F^(-)rarr ii) 6NaOH" (...
Text Solution
|
- Give reasons: i) Cu^(+2) (aq) is more stable than Cu^(+) ii) Ioni...
Text Solution
|
- Write the two steps involved in the commercial process of converting M...
Text Solution
|
- Give the structure of chromate ion?
Text Solution
|
- With the help of Valence Bond theory account for hybridisation, geomet...
Text Solution
|
- a) What type of isomerism is exhibited by the square planar complex of...
Text Solution
|
- b) How is a metal-carbon pi bond formed in metal carbonyls?
Text Solution
|