Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 2)
SUNSTAR PUBLICATION|Exercise PART - C|10 VideosII PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 1)
SUNSTAR PUBLICATION|Exercise PART - D|25 VideosII PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 3)
SUNSTAR PUBLICATION|Exercise PART - D|30 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 2)-PART - D
- c) Write Nernst equation for the cell represented as: Mg(s)|Mg((aq))^(...
Text Solution
|
- Show that for a first order reaction, t(99.9%)=10t(1//2).
Text Solution
|
- b) In the graph drawn what does A and the shaded region B represent?
Text Solution
|
- Name the phenomenon/process involved i) mixing of hydrated ferric ox...
Text Solution
|
- Mention any two characteristic of enzyme catalysis.
Text Solution
|
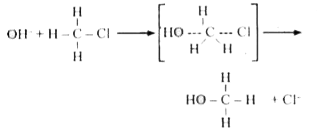
- a) Write the equation for SN^(2) mechanism between CH(3)Cl and -OH. Wh...
Text Solution
|
- b i) Aryl halide with sodium in dry ether undergoes Fitting reaction. ...
Text Solution
|
- a) What is the organic compound formed in the following :
Text Solution
|
- b) Give reason: - i) o-nitrophenol and p-nitrophenol can be separate...
Text Solution
|
- a) Write the structure of P and Q? Name the reaction that gives the pr...
Text Solution
|
- Explain decarboxylation reaction with an example.
Text Solution
|
- a) Name the products X and Y?
Text Solution
|
- b) Between methyl amine and ammonia which has lower pK(b) value and wh...
Text Solution
|
- Name the final product of ammonolysis of an alkyl halide.
Text Solution
|
- Draw the Haworth structure of beta-(D) fructofuranose.
Text Solution
|
- b) i) Pentaacetate of glucose does not react with hydroxyl amine. What...
Text Solution
|
- What major molecular shape does the tertiary structure of protein lead...
Text Solution
|
- What is a homopolymer? Give an example.
Text Solution
|
- Write the equation for the formation of the polymer by the interaction...
Text Solution
|
- c) Molecular mass of polymers are expressed as an average. Give reason
Text Solution
|