Text Solution
Verified by Experts
Topper's Solved these Questions
SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2018
SUNSTAR PUBLICATION|Exercise PART - D|28 VideosSUPPLEMENTARY EXAM QUESTION PAPER JULY - 2018
SUNSTAR PUBLICATION|Exercise PART - B|8 VideosK-CET-CHEMISTRY-2019
SUNSTAR PUBLICATION|Exercise Question|60 VideosSUPPLEMENTARY EXAM QUESTION PAPER JULY - 2015
SUNSTAR PUBLICATION|Exercise PART - D|30 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2018-PART - C
- What is saponification? Give the equation to form sodium stearate by t...
Text Solution
|
- Draw a neat labelled diagram of electrolytic cell used in the extracti...
Text Solution
|
- Write the equations with conditions for the manufacture of nitric acid...
Text Solution
|
- Complete the following equations: (i) SO(2) + Cl(2) overset("charcoa...
Text Solution
|
- Write any two anomalous properties of fluorine.
Text Solution
|
- Give an equation for the reaction of chlorine with hydrogen sulphide.
Text Solution
|
- Transition metals show catalytic property: Give reasons.
Text Solution
|
- Between Cu((aq))^(2+) and Cu((aq))^(+) which is more stable?
Text Solution
|
- Write the balanced equations in the manufacture of potassium dichromat...
Text Solution
|
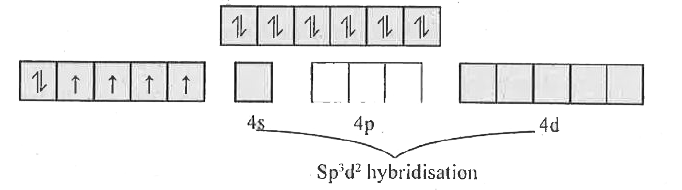
- On the basis of VBT explain the hybridization, geometrical shape and m...
Text Solution
|
- What is an ambidentate ligand ? Name the type of structural isomerism ...
Text Solution
|