Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2018-PART - D
- Write the IUPAC name of K(2)[Zn(OH)(4)].
Text Solution
|
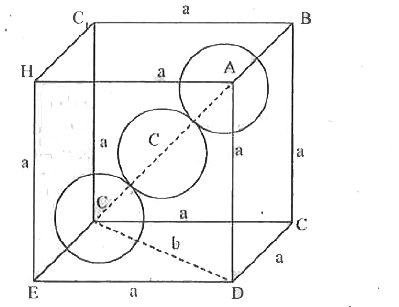
- a) Calculate the packing efficiency of particles in a body centred cub...
Text Solution
|
- What is Schottky defect ?
Text Solution
|
- 5.8 g of non - volatile, non - electrolyte solute was dissolved in 100...
Text Solution
|
- Mention any two differences between ideal and non-ideal solutions.
Text Solution
|
- Calculate the e.m.f. of the cell in which the following reaction takes...
Text Solution
|
- State the Faraday's first law of electrolysis. How many Faraday of ele...
Text Solution
|
- Derive an integrated rate equation for the rate constant of a zero ord...
Text Solution
|
- Draw a graph of potential energy V/S reaction co - ordinates showing t...
Text Solution
|
- Write any two characteristics of chemical adsorption.
Text Solution
|
- What is Brownain movement ? What is the cause for it ?
Text Solution
|
- What is homogenous catalysis? Give an example.
Text Solution
|
- Write equations for the steps in S(N)1 mechanism of conversion of tert...
Text Solution
|
- Explain Wurtz-Fitting reaction with equation.
Text Solution
|
- CH(3)Cl + NaI overset("Dry acetone")rarr CH(3)I + NaCl. Name the above...
Text Solution
|
- Write the mechanism of acid catalysed dehydration of ethanol to ethene...
Text Solution
|
- How does anisole react with bromine in ethanoic acid ? Give equation.
Text Solution
|
- Complete the following equations : (i) (iii) CH(3)COONa overset(N...
Text Solution
|
- Explain esterification reaction with an example.
Text Solution
|
- How is methylamine prepared by Hoffmann bromamide degradation reaction...
Text Solution
|