Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MAXIMUM PUBLICATION-CHEMICAL BONDING-EXAMPLE
- Draw the electron dot diagram of the transference of electron sodium a...
Text Solution
|
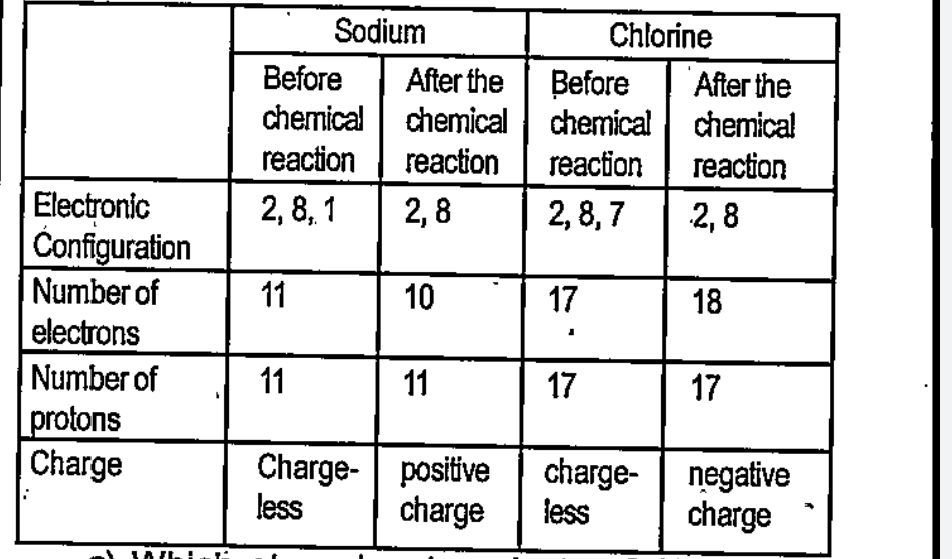
- Complete Table 2.3 by examining the arrangement of electrons before an...
Text Solution
|
- Complete Table 2.3 by examining the arrangement of electrons before an...
Text Solution
|
- Electron tranfer during the formation of sodium chloride can be writte...
Text Solution
|
- Define Ionic Bond?
Text Solution
|
- How the ionic bond formation of sodium oxide is represented? [Hint: ...
Text Solution
|
- Draw the electron dot diagram of following compounds. [Hint: Atomic No...
Text Solution
|
- Define ionic compounds?
Text Solution
|
- Write the atomic number of flourine?
Text Solution
|
- The electronic configuration of Flourine
Text Solution
|
- How many electrons are required for one flourine atom to attain the oc...
Text Solution
|
- Is there a possibility of transferring electrons from one flourine ato...
Text Solution
|
- How can the two flourine atoms attain an octect arrangement?
Text Solution
|
- The manner in which the two flourine atoms in a flourine molecule unde...
Text Solution
|
- What happens during the formation of flourine molecule electron transf...
Text Solution
|
- How many pairs of electrons are shared?
Text Solution
|
- How covalent bonds are formed?
Text Solution
|
- How single bonds are formed?
Text Solution
|
- Write down the electronic configuration?
Text Solution
|
- Draw the electron dot diagram of the formation of chlorine molecule by...
Text Solution
|
 .
.