Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MAXIMUM PUBLICATION-CHEMICAL BONDING-EXAMPLE
- In the formation of hydrogen chloride, how many electron pairs are sha...
Text Solution
|
- What will be the valency of each atom?
Text Solution
|
- Why does the number of chlorine atoms differ in these compounds? Try t...
Text Solution
|
- Complete the table given below and answer the following questions (sym...
Text Solution
|
- Complete the table given below and answer the following questions (sym...
Text Solution
|
- Complete the table given below and answer the following questions (sym...
Text Solution
|
- Electronegativity values of some elements are given. Using these value...
Text Solution
|
- Electronegativity values of some elements are given. Using these value...
Text Solution
|
- Electronegativity values of some elements are given. Using these value...
Text Solution
|
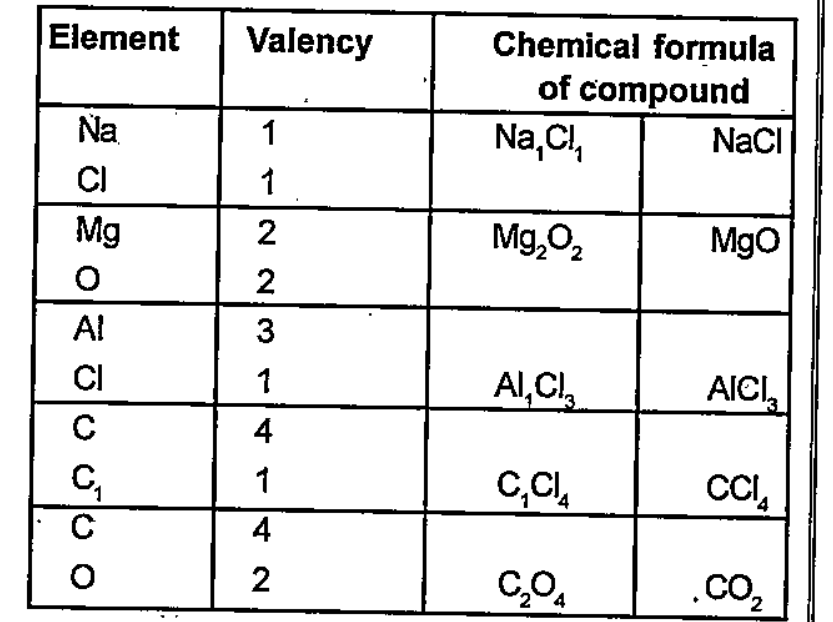
- Some elements and their valencies are given Write the chemical formu...
Text Solution
|
- Some elements and their valencies are given Write the chemical formu...
Text Solution
|
- Some elements and their valencies are given The chemical formula of ...
Text Solution
|
- Examine the following chemical equations and answer the questions (Hin...
Text Solution
|
- Examine the following chemical equations and answer the question. (Hi...
Text Solution
|
- Examine the following chemical equations and answer the questions. (H...
Text Solution
|
- Electronegativity values of some elements are given. Using these value...
Text Solution
|
- Draw the electron dot diagram of chemical bonds in methane (CH4) and e...
Text Solution
|
- P,Q,R,S are four elements. Their atomic numbers are 8,17,12 and 16 res...
Text Solution
|
- P,Q,R,S are four elements. Their atomic numbers are 8,17,12 and 16 res...
Text Solution
|
- P,Q,R,S are four elements. Their atomic numbers are 8,17,12 and 16 res...
Text Solution
|
 .
.