Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MAXIMUM PUBLICATION-REDOX REACTIONS AND RATE OF CHEMICAL REACTIONS-EXAMPLE
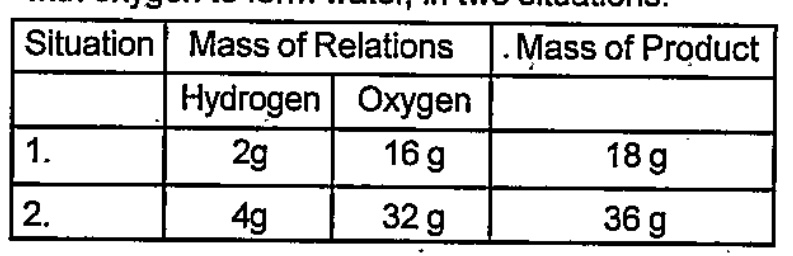
- What is the relation between the total mass of the reactants and the t...
Text Solution
|
- A piece of magnesium is burned in air. What do you observe ?
Text Solution
|
- A piece of magnesium is burned in air. What is the white powder form...
Text Solution
|
- A piece of magnesium is burned in air. Which are the reactants here ...
Text Solution
|
- A piece of magnesium is burned in air. Which is the product ?
Text Solution
|
- Is the number of atoms of each element equal on both sides ?
Text Solution
|
- How will you represent two molecules of magnesium oxide ?
Text Solution
|
- Now, is the number of magnesium atoms equal on both sides ?
Text Solution
|
- Is the total number of atoms of each element in the molecules present ...
Text Solution
|
- What is balancing of equations ?
Text Solution
|
- Balance the chemical equation H2 +O2 rarrH2O
Text Solution
|
- Balance the chemical equation Al +O2 rarrAl2O3
Text Solution
|
- Balance the chemical equation H2 + O2 rarr H2O
Text Solution
|
- Some chemical equations are given below. Note down the number of react...
Text Solution
|
- The electronic configuration of magnesium and chlorine are 2, 8, 2 and...
Text Solution
|
- Let us complete the equation for this process, Mg rarr Mg^(2+)+ .......
Text Solution
|
- How many electrons are accepted by each chlorine atom ? What will be t...
Text Solution
|
- Complete the equation of this process. Cl + 1e^-)rarr .............
Text Solution
|
- What are oxidation and reduction ?
Text Solution
|
- In the above chemical reaction, Which atom is oxidised ? 2SO2 + O2 ...
Text Solution
|