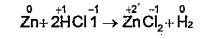Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MAXIMUM PUBLICATION-REDOX REACTIONS AND RATE OF CHEMICAL REACTIONS-EXAMPLE
- Analyse oxidation numbers in the given equation and list the oxidising...
Text Solution
|
- The Oxidised atom is …….
Text Solution
|
- The oxidation number of hydrogen increases/ decreases from ……… to ……....
Text Solution
|
- How do you determine the oxidation number of sulphur in H2SO4 ?
Text Solution
|
- Find the oxidation number of Mn in KMnO4 (oxidation number of K is +1,...
Text Solution
|
- Find the oxidation number of Mn in MnO2
Text Solution
|
- Find the oxidation number of Mn in Mn2O3
Text Solution
|
- Find the oxidation number of Mn in Mn2O7
Text Solution
|
- What is Redox reaction ?
Text Solution
|
- What are the methods usually adopted to make firewood burn faster ?
Text Solution
|
- Describe an experiment to prove that nature of the reactants affect th...
Text Solution
|
- Write an experiment to prove that concentration of reactants affect th...
Text Solution
|
- Write your observation Test tube 2 : ……………
Text Solution
|
- Why rate of reaction increases when concentration increases ?
Text Solution
|
- What is the relation between rate of reaction and surface area. Write ...
Text Solution
|
- Is there any difference in the rate of reaction in the two beakers ?
Text Solution
|
- What about the concentration of acid in both the reactions ?
Text Solution
|
- What is the change in the rate of collision when surface area increas...
Text Solution
|
- Why rate of reaction increases when surface area increases ?
Text Solution
|
- Write an experiment to prove the relation between temperature and rate...
Text Solution
|