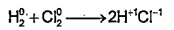Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MAXIMUM PUBLICATION-REDOX REACTIONS AND RATE OF CHEMICAL REACTIONS-EXAMPLE
- From the given chemical equation identify the oxidising agent and the ...
Text Solution
|
- In the formation of hydrogen chloride does the oxidation number of hyd...
Text Solution
|
- Whether the oxidation number of chlorine decrease or increase ?
Text Solution
|
- What is meant by Redox reaction ?
Text Solution
|
- Calculate the oxidation number of 's' in H2SO4 ?
Text Solution
|
- Find the oxidation number of the ions in ionic compound ?
Text Solution
|
- Some chemical equations are given below 1) C+O2 rarr CO2 2) CH4+2O...
Text Solution
|
- Some chemical equations are given below 1)C+02 rarr CO2 2)CH4+2O2 ...
Text Solution
|
- Some chemical equations are given below 1)C+O2 rarr CO2 2)CH4+2O2 ...
Text Solution
|
- The chemical reaction between marble and dilute HCl is given CaCO3+2HC...
Text Solution
|
- The chemical reaction between marble and dilute HCl is given CaCO3+2HC...
Text Solution
|
- Sulphur pieces do not react with cold concentrated nitric acid . But s...
Text Solution
|
- Sulphur pieces do not react with cold concentrated nitric acid . But s...
Text Solution
|
- Small amounts of phosphoric acid is usually added to hydrogen peroxide...
Text Solution
|
- Small amounts of phosphoric acid is usually added to hydrogen peroxide...
Text Solution
|
- Small amounts of phosphoric acid is usually added to hydrogen peroxide...
Text Solution
|
- Find the oxidation number of the elements which are underlined in the ...
Text Solution
|
- Find the oxidation number of the elements which are underlined in the ...
Text Solution
|
- Find the oxidation number of the elements which are underlined in the ...
Text Solution
|
- Find the oxidation number of the elements which are underlined in the ...
Text Solution
|