Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MAXIMUM PUBLICATION-CLASSIFICATION OF ELEMENTS AND THE PERIODIC TABLE-EXAMPLE
- Are you familiar with the Bohr model of an atom? See the Bohr model of...
Text Solution
|
- Are you familiar with the Bohr model of an atom? See the Bohr model of...
Text Solution
|
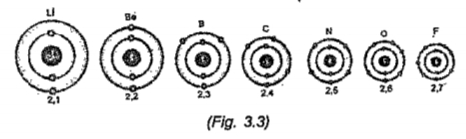
- The representation of Bohr model of elements with atomic number 3 to 9...
Text Solution
|
- What happens to the nuclear charge with increase in atomic number?
Text Solution
|
- You have understood how sodium chloride is formed by combining sodium ...
Text Solution
|
- How the ions are formed?
Text Solution
|
- Define ionization energy?
Text Solution
|
- What are the factors affecting the ionisation energy?
Text Solution
|
- When the size of an atom increases, does the attraction of the nucleus...
Text Solution
|
- Then what is the change in ionization energy?
Text Solution
|
- Can you find out how ionization energy changes as we move from top to ...
Text Solution
|
- What is the general trend in the variation of ionisation energy on mov...
Text Solution
|
- Find how ionization energy changes with increase in nuclear charge?
Text Solution
|
- Define electronegativity?
Text Solution
|
- How size of an atom influence the electronegativity?
Text Solution
|
- What is the basis for the chemical properties of metals and non metals...
Text Solution
|
- What is relationship between metallic character and the size of an ato...
Text Solution
|
- How do the metallic character and non metallic character vary while mo...
Text Solution
|
- Don't you think that there is a relationship between ionization energy...
Text Solution
|
- Then what about those having the low ionization energy?
Text Solution
|