Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MAXIMUM PUBLICATION-MODEL PAPER 2-EXAMPLE
- What are the characteristics of transition elements?
Text Solution
|
- What are redox reactions?
Text Solution
|
- What is the relation between surface area and rate of chemical reactio...
Text Solution
|
- The atomic number of two elements are given below. Mg = 12, O = 8 W...
Text Solution
|
- The atomic number of two elements are given below.Mg = 12,O = 8 Whic...
Text Solution
|
- The atomic number of two elements are given below.Mg = 12,O = 8 Draw...
Text Solution
|
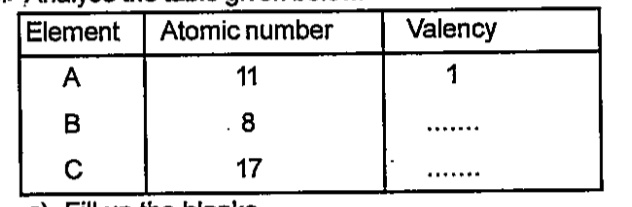
- Analyse the table given below. Fill up the blanks
Text Solution
|
- Analyse the table given below. Write the chemical formula of the com...
Text Solution
|
- Which of the following chemical equation are balanced? H2+O2rarrH2O ...
Text Solution
|
- Balance the unbalanced chemical equations.
Text Solution
|
- There are 4 electrons in the M shell of an element. Write the electr...
Text Solution
|
- What Is atomic number?
Text Solution
|
- Find out the period and group of the element in the periodic table wit...
Text Solution
|
- An incomplete form of the periodic table Is given below.(symbols are n...
Text Solution
|
- An incomplete form of the periodic table Is given below.(symbols are n...
Text Solution
|
- Find out the oxidation number of 'S' in the following compounds. (Oxi...
Text Solution
|
- Find out the oxidation number of 'S' in thefollowing compounds. (Oxida...
Text Solution
|
- Analyse the following chemical equation and find out the element under...
Text Solution
|
- Sodium chloride Is an ionic compound and wax is a covalent compound. H...
Text Solution
|
- Write the main postulates of the Bohr model of atom.
Text Solution
|