A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
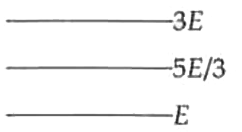
- The figure shows the energy level of certain atom. When the electron d...
Text Solution
|
- The energy levels of a cartain atom are shown in figure. If a photon o...
Text Solution
|
- The wavelength of radiation emitted due to transition of electron from...
Text Solution
|
- The electron of hydrogen atom is excited to certain level. When the el...
Text Solution
|
- The figure shows the energy level of certain atom. When the electron d...
Text Solution
|
- सलग्न चित्र में किसी का ऊर्जा -स्तर प्रदर्शित है । जब परमाणु 2E से E ...
Text Solution
|
- When the electron in a hydrogen atom jumps from the second orbit to th...
Text Solution
|
- The short wavelength electromagnetic wave emitted by nuclei are called
Text Solution
|
- दिया गया चित्र किसी परमाणु के ऊर्जा स्तरों को दर्शाता है। जब इलेक्ट्रॉ...
Text Solution
|