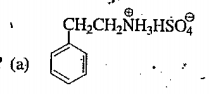
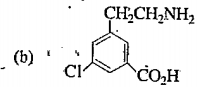
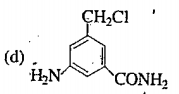
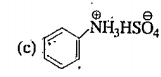A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
UNITED BOOK HOUSE-2015 QUESTION PAPER -EXERCISE
- Which of the following compex ions has no 'd' electron (s) in the cent...
Text Solution
|
- Which of the following is produced when benzene diazonium chloride is ...
Text Solution
|
- An organic compound (A) is soulbe in water. Its aqueous solution liber...
Text Solution
|
- Which of the following is a polymide polymer?
Text Solution
|
- For which of the following purpose sodium benzoate is used?
Text Solution
|
- What is the number of unit cells in 6.4 g of X (atomic mass=64) (X cry...
Text Solution
|
- Which of the following is the most effective in bringing about the coa...
Text Solution
|
- Which of the following is the correct electronic configura-tion of Ni ...
Text Solution
|
- A molecule has the following structure. Which one is the IUPAC name of...
Text Solution
|
- Which of the following will respond to Cannizzaro reaction?
Text Solution
|
- In which of the following peptide bond is present?
Text Solution
|
- Among the following which is artificial sweetening agent?
Text Solution
|
- Give an example of a soap and indicate its nonpolar and polar parts.
Text Solution
|
- What is the magnetic character of Cu^+ ion?
Text Solution
|
- Which trivalent ion among lanthanoids has the largest size?
Text Solution
|
- What happens when NaCI solution is added to a gold sol?
Text Solution
|
- The specific conductance of a 0.20 mol L^-1 solution of KCI at 300 K i...
Text Solution
|
- Write down the reactions occuring at the two electrodes when current i...
Text Solution
|
- 2.00 g of non=electrolyte solute dissolved in 100 g of benzene lowered...
Text Solution
|
- Explain, with reason, the nature of variation of the vapour pressure o...
Text Solution
|